Complete Guide for Thin Layer Chromatography
Introduction
In the field of analytical chemistry, chromatography is a widely used technique to separate and identify different components in a mixture. Thin Layer Chromatography (TLC) is one of the simplest and most efficient chromatographic techniques. It is a powerful tool for qualitative analysis and is extensively used in various industries, including pharmaceuticals, forensics, food, and environmental analysis. This article will provide a comprehensive guide to Thin Layer Chromatography, exploring its principles, applications, experiment procedures, and more.
What is Thin Layer Chromatography?
Thin Layer Chromatography (TLC) is a chromatographic separation technique in which a stationary phase, typically a thin layer of adsorbent material (e.g., silica gel or alumina) coated on a glass, metal, or plastic plate, is used to separate the components of a sample based on their affinity to the stationary phase and mobile phase.

The mobile phase, which is a solvent or a mixture of solvents, moves up the plate by capillary action, carrying the sample components along with it. As the mobile phase travels up the plate, the different components of the sample interact differently with the stationary phase, leading to their separation based on their affinities and interactions.
Thin Layer Chromatography Principle
Thin layer chromatography principle defined as it is the differential migration of the components of a mixture over the stationary phase due to their varying affinities for the stationary phase and mobile phase. The components that have stronger interactions with the stationary phase will move more slowly, while those with weaker interactions will move faster.
When the TLC plate is developed, the distance traveled by each component is compared to the distance traveled by the solvent front. The ratio of the distance traveled by the component to the distance traveled by the solvent front is known as the Rf value (Retention Factor). Rf value is specific for each compound and helps in the identification of unknown substances by comparing them to known reference compounds.
Diagram of Thin Layer Chromatography
The Thin Layer Chromatography Diagram and Thin Layer Chromatography experimental setup is shown below,
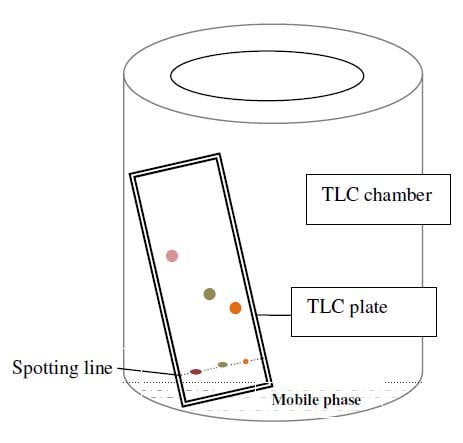
Thin Layer Chromatography Experiment and Procedure
Performing a TLC experiment involves several essential steps:
- Preparation of the TLC Plate: The stationary phase, typically silica gel or alumina, is mixed with a binder and spread evenly as a thin layer on a glass or plastic plate. The plate is then heated to activate the binding and ensure a uniform surface.
- Sample Application: A small spot of the sample is applied to the bottom of the TLC plate using a capillary tube or a micropipette. Care must be taken not to overload the plate to avoid smearing or overlapping spots.
- Development: The TLC plate is placed vertically in a developing chamber containing the mobile phase. The mobile phase rises up the plate through capillary action, carrying the sample components along with it. The chamber is covered to prevent evaporation of the mobile phase.
- Visualization: Once the mobile phase front reaches a specific distance from the top of the plate, the plate is taken out of the chamber. The components on the plate are then visualized by exposing the plate to ultraviolet (UV) light or by using chemical reagents that react with specific compounds to form visible spots.
- Rf Value Calculation: The Rf value of each component is calculated using the formula: Rf = (Distance traveled by the component) / (Distance traveled by the solvent front).
- Interpretation and Analysis: The Rf values are compared to reference values for known compounds to identify the components in the sample.
Difference Between Thin Layer Chromatography and Paper Chromatography
| Feature | Thin Layer Chromatography (TLC) | Paper Chromatography |
| Principle | Stationary phase on a thin layer of silica gel or alumina coated on a plate | Stationary phase on a strip of filter paper |
| Support material | Rigid plate (usually glass, plastic, or aluminum) | Flexible strip of filter paper |
| Sample application | Applied as small spots near one edge of the plate | Applied as small spots near one end of the paper strip |
| Mobile phase | Solvent moves up the plate through capillary action | Solvent moves up the paper through capillary action |
| Separation mechanism | Based on differential adsorption and partitioning of components | Based on differential adsorption and partitioning of components |
| Separation efficiency | Generally provides better resolution and separation | Resolution may be lower than TLC |
| Speed of separation | Faster due to the thin layer and efficient solvent movement | Slower due to the thicker paper and slower solvent movement |
| Sensitivity | More sensitive, detects even minor differences in components | May be less sensitive |
| Visualization of results | Usually UV light or staining agents are used | Dyes or color-developing reagents are used |
| Applications | Analyzing mixtures, identifying compounds, and monitoring reactions | Suitable for educational purposes and separation of simple mixtures |
| Quantitative analysis | Often used for qualitative analysis | Not commonly used for quantitative analysis |
| Cost | Generally more expensive due to specialized plates | Cost-effective due to the simplicity of materials |
| Practicality | Commonly used in research and analytical laboratories | Widely used in educational settings and for simple separations |
Applications of Thin Layer Chromatography
Thin Layer Chromatography finds wide applications in different industries and laboratories, including:
- Pharmaceuticals: For analyzing drug purity and identifying unknown compounds in pharmaceutical formulations.
- Forensics: In the analysis of crime scene samples and identifying illegal substances.
- Food Industry: For quality control and detecting adulterants in food products.
- Environmental Analysis: To analyze pollutants and contaminants in environmental samples.
- Chemical Research: In research labs to study reaction progress and purity of compounds.
Pros and Cons of Thin Layer Chromatography
The Advantages and disadvantages of Thin Layer Chromatography are listed below,
Advantages of Thin Layer Chromatography
- Quick and easy to perform.
- Requires minimal sample preparation.
- Cost-effective compared to other chromatographic techniques.
- High sample throughput.
- Suitable for both qualitative and quantitative analysis.
Disadvantages of Thin Layer Chromatography
- Less resolution compared to some other chromatographic techniques.
- Limited load capacity for larger samples.
- Sensitivity may not be as high as other methods.
- Overlapping bands may occur in complex mixtures.
Final Notes
Thin Layer Chromatography is a versatile and indispensable analytical technique in the field of chemistry. Its simplicity, speed, and effectiveness make it a valuable tool for researchers, analysts, and scientists across various industries. By harnessing the principles of partitioning, TLC enables the separation and identification of components in a mixture with remarkable efficiency.
We trust that our articles have adequately addressed all about tlc chromatography like thin layer chromatography principle, application of thin layer chromatography, and advantages, and disadvantages of thin layer chromatography. Tutoroot stands as an educational establishment providing interactive online classes. To experience our live interactive sessions for NEET online tuition and explore various message boards, you are welcome to schedule a complimentary demo.
FAQs
What is the principle of thin-layer chromatography?
The principle of Thin Layer Chromatography is based on the differential migration of components in a mixture due to their varying affinities for the stationary and mobile phases.
Which solvent is used in TLC?
The choice of solvent in TLC depends on the nature of the sample and the components to be separated. Common solvents include ethyl acetate, methanol, and hexane.
Is the TLC plate polar or nonpolar?
The TLC plate is typically polar due to the presence of the adsorbent material, such as silica gel or alumina, which has polar properties.
What are the two phases of TLC?
The two phases in TLC are the stationary phase, represented by the adsorbent-coated plate, and the mobile phase, which is the solvent used for developing the plate.
Can TLC be used quantitatively?
Yes, TLC can be used for quantitative analysis by comparing the Rf values of known and unknown samples. However, it is more commonly used for qualitative purposes.
