What is Paper Chromatography? – Experiment, Diagram, Applications
Introduction to Paper Chromatography
Paper chromatography is a powerful analytical technique used to separate and identify different components of a mixture based on their affinity for a stationary phase (paper) and a mobile phase (solvent). This versatile method has found extensive applications in various fields, from chemistry laboratories to forensic investigations. In this article, we will explore the principles, types, diagrams, experiments, procedures, and applications of paper chromatography.
What is Paper Chromatography?
Paper chromatography is a type of chromatographic technique that employs a porous paper strip or sheet as the stationary phase and a liquid solvent as the mobile phase. This technique takes advantage of the differential solubility and adsorption properties of the components in a mixture, enabling their separation and identification.
Paper Chromatography Principle
The principle of paper chromatography lies in the concept of partitioning. When a mixture is applied near one end of the paper strip, the mobile phase (solvent) moves through capillary action, carrying the mixture along with it. As the solvent travels up the paper, it interacts with the compounds in the mixture. Depending on their solubility and affinity for the paper, the components are selectively carried at different rates. This differential movement allows the separation of the mixture into its individual components.
How Paper Chromatography Principle Works?
Well, it relies on a simple principle: different substances in a mixture will move at different speeds through a piece of special paper when they’re dissolved in a liquid. This paper is called chromatography paper, and it’s designed to absorb the liquid and let it travel.
- Sample Application: First, you take a small drop of your mixture and place it near the bottom of the chromatography paper. This is where the magic begins.
- Developing Solvent: Next, you dip the bottom of the paper into a liquid, called the developing solvent. This liquid moves up the paper by a process called capillary action, and it carries the different components of your mixture along with it.
- Separation Time: As the solvent travels up the paper, it carries the mixture’s components with it. But here’s the cool part – because each substance in the mixture interacts with the paper and solvent differently, they move at different speeds. This causes them to spread out along the paper, creating colorful bands.
- Identification: Once the solvent reaches the top of the paper, you’ve got a beautiful chromatogram. Now, you can see the different components of your mixture separated out. It’s like having a secret code that tells you what’s in your mixture!
Types of Paper Chromatography
There are several types of paper chromatography, each with its own specific applications and techniques. Here are some commonly used variations:
- Ascending Paper Chromatography: In this method, the solvent is allowed to rise up the paper strip by capillary action, separating the mixture components as it progresses. It is particularly useful for separating small molecules and organic compounds.
- Ascending Thin-Layer Chromatography (TLC): Similar to ascending paper chromatography, but the stationary phase is a thin layer of silica gel or alumina on a glass or plastic plate. TLC offers faster separation and is commonly used in qualitative analysis.
- Descending Paper Chromatography: Unlike ascending paper chromatography, the solvent is applied at the top and allowed to flow downwards, carrying the mixture components. This technique is advantageous when analyzing non-volatile substances or those that are more soluble in the solvent.
- Two-Dimensional Paper Chromatography: This method involves the sequential application of solvents in two directions, allowing better separation and analysis of complex mixtures.
- Preparative Paper Chromatography: In this technique, larger quantities of separated components are obtained for further analysis or use. It is commonly used for isolating pure compounds.
Paper Chromatography Diagram
To help visualize the paper chromatography process, refer to the following diagram:
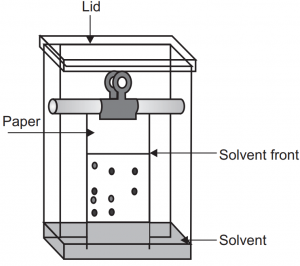
The diagram illustrates the setup of paper chromatography, with the mixture applied near one end of the paper strip. The solvent migrates through the paper, carrying the components at different rates. As the solvent progresses, distinct bands or spots corresponding to each component are formed, allowing easy identification.
Paper Chromatography Experiment and Procedure
Carrying out a paper chromatography experiment requires careful attention to detail. Here is a general procedure to follow:
- Prepare the mixture: Obtain a small quantity of the mixture to be analyzed and ensure it is properly dissolved or suspended in a suitable solvent.
- Prepare the paper strip: Cut a piece of chromatography paper to the desired size. It is crucial to handle the paper strip with clean hands or gloves to avoid contamination.
- Apply the mixture: Using a capillary tube or dropper, carefully apply a small spot or line of the mixture near one end of the paper strip. Allow it to dry completely to avoid smudging.
- Prepare the solvent: Choose an appropriate solvent based on the nature of the mixture components. Pour the solvent into a suitable container, ensuring it is deep enough to allow the solvent front to reach the desired height on the paper strip.
- Perform the chromatography: Immerse the paper strip vertically into the container, ensuring the mixture spot is above the solvent level. Cover the container to prevent evaporation. Capillary action will cause the solvent to rise through the paper, separating the components. The developed chromatogram can take anywhere from minutes to hours.
- Interpret the results: Once the solvent front reaches the desired distance (usually about 2/3 of the paper strip height), remove the paper strip from the container and allow it to air dry. Observe the separated components as distinct bands or spots. Identify and mark them as needed for further analysis.
Applications of Paper Chromatography
Paper chromatography finds extensive applications in various fields due to its simplicity, versatility, and cost-effectiveness. Some notable applications include:
- Food and Beverage Analysis: Paper chromatography is used to analyze food additives, dyes, and contaminants. It enables the identification and quantification of components in complex food matrices.
- Pharmaceutical Industry: This technique plays a crucial role in the quality control and analysis of pharmaceutical substances. It aids in drug formulation development, stability testing, and detection of impurities.
- Forensic Science: Paper chromatography is employed to analyze ink compositions and differentiate between counterfeit and genuine documents. It helps forensic experts determine the origin of ink samples and provide valuable evidence in investigations.
- Environmental Analysis: By separating and analyzing environmental pollutants, such as pesticides and heavy metals, paper chromatography contributes to environmental monitoring and assessment.
- Biochemical Analysis: Paper chromatography is used for the separation and identification of amino acids, sugars, vitamins, and other biological compounds. It plays a vital role in protein characterization and the detection of metabolic disorders.
Final Notes
Paper chromatography is an indispensable technique in the field of analytical chemistry. Its ability to separate and identify components in mixtures has proven invaluable across various scientific disciplines. By understanding the principles, types, experiment procedures, and applications of paper chromatography, scientists and researchers can harness its power to unlock valuable insights from complex mixtures.
This is all about Paper chromatography, hope the above explanation gives a good clarity on Paper Chromatography. As well we gave an in-depth explanation of the Paper Chromatography experiment and procedure. To learn much more important concepts in a simpler manner visit our blog page there are many difficult concepts in chemistry, to learn those concepts in an easy manner Tutoroot Online home tuitions will help a lot. Click here to get a FREE DEMO session from an expert faculty to learn about the teaching methodology.
FAQs
What is the principle of paper chromatography?
The principle of paper chromatography is based on the differential solubility and affinity of mixture components for both the stationary phase (paper) and the mobile phase (solvent). This differential interaction causes the components to move at different rates, leading to their separation.
What are the 3 main steps of paper chromatography?
The three main steps of paper chromatography are:
- Mixture application: Applying a small spot or line of the mixture on the paper strip.
- Solvent migration: Allowing the solvent to rise through the paper strip, carrying the mixture components.
- Results interpretation: Observing and analyzing the separated components on the developed chromatogram.
