Laws of Thermodynamics 2024
The Laws of Thermodynamics help individuals define fundamental physical quantities such as temperature, entropy, and energy, which play a crucial role in characterizing thermodynamic systems at thermodynamic equilibrium. This helps in finding out how each of these quantities generally behaves under different types of circumstances or changes. It is important to understand various concepts or chapters in Physics, as they are directly derived or indirectly connected to the Laws of Thermodynamics.
In this article, let us understand the laws of thermodynamics and the properties of thermodynamics.
What is Thermodynamics?
Thermodynamics is a specialized study of heat and temperature and their various dimensions and concepts. These aspects related to heat and temperature, under thermodynamics and the inter-conversion of heat and other forms of energy form very interesting and crucial study in physics. There are four laws of thermodynamics that determine and drive their quantities and explain their quantitative nature. The credit to coin the word, thermodynamics goes to William Thomson, who gave this name in 1749.
Types of Laws of Thermodynamics
So, what are the laws of thermodynamics?
First of all, the laws of thermodynamics help us understand how thermal energy can be converted to or from other forms of energy. They explain the effects on the matter owing to this process. As we know, thermal energy comes from heat that is generated by the movement of tiny particles within an object. The speed of the movement of these particles determines the quantity of generation of heat.
There are multiple laws under Thermodynamics, each explaining how a different quantity performs in different situations. These are:
- Zeroth Law of Thermodynamics
- First Law of Thermodynamics
- Second Law of Thermodynamics
- Third Law of Thermodynamics
Zeroth Law of Thermodynamics
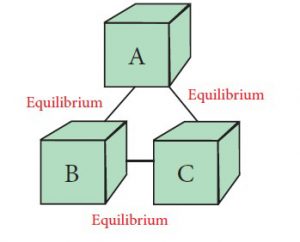
Under the law of thermodynamics, Zeroth Law of Thermodynamics explains that, two bodies that are individually in equilibrium with a third body will also be in thermal equilibrium with each other.
From the diagram, as you can see, object B and Object B are maintaining equilibrium. At the same time, Object A is also in equilibrium with the two objects. This diagram can be pictured as two water cups and a thermometer, which is used to measure the temperature of water in either of these cups.
First Law of Thermodynamics
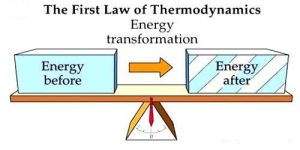
Let us understand the first law of thermodynamics. The Law of Conservation of Energy, or the First Law of Thermodynamics, states that energy can neither be created nor destroyed, instead it can only be transferred from one form to another.
To explain the first law of thermodynamics in much more detail:
For instance, if you switch on a light, you can absorb the electrical energy that is converted to light.
This can also be absorbed in plants when they convert the radiant energy of sunlight into starch or food by producing chemical energy through the photosynthesis process.
The first law of thermodynamics can also be explained as follows:
When there is an isolated system, the total energy of the system remains constant, even if the energy is transformed from in one form to the other. when the system is not in the state of isolation, the development or change in a system’s internal energy (ΔU) equals the difference between the heat Q that is added to the system from its periphery and the work W performed by the system on its peripheral areas. This means ΔU = Q − W. This summarizes the first law of thermodynamics in a formulaic way.
Second Law of Thermodynamics
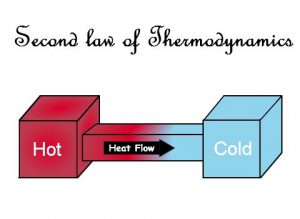
The Second Law of Thermodynamics suggests that the entropy in an isolated system will always increase. As the isolated systems have a high tendency to evolve towards thermal equilibrium, which in turn means that the state of entropy of the system will be at maximum. This is mainly because the entropy of the universe only increases.
Further to explain the second law of thermodynamics: For instance, when a car is placed outside, it will inevitably get dirty and messy. However, if the individual cleans his car, then it will become tidy, and the process will result in the rise of the entropy, in the surroundings of the car.
Let us explain the second law of thermodynamics as follows:
We know that in the colder regions, heat does not transfer to a warmer place. Similarly, the heat at a given degree cannot be changed into work in its entirety. Thus, the entropy or the measure of the disorder of the material of any closed system, or heat energy per unit temperature, goes up in a given period of time and touches a maximum value. In this way, the closed systems move to attain a state of equilibrium where the entropy reaches a maximum and there will not be any energy left to perform work. This summarizes the second law of thermodynamics.
Third Law of Thermodynamics
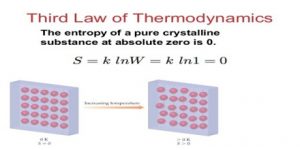
Now, let us understands the third law of thermodynamics. When the temperature of a system reaches absolute zero, then at the same time the entropy of a system will reach a constant value. For instance, the entropy of a pure crystalline substance is zero when it is at absolute temperature.
These are some of the conditions where the Third Law of Thermodynamics is applicable.
- When the water is heated to its boiling level, the vapor is released in gaseous form. Because of the high temperature, the particles in the steam move randomly, which is a clear example of high entropy.
- Similarly, if the water is cooled, it is converted into ice. Then the particles in the ice are further restricted, which means the entropy is further decreasing.
Terms of Thermodynamics
Now that you have understood the four thermodynamics laws and formulas, let us talk about the different terms of thermodynamics in brief below. Let us also cover the properties of thermodynamics.
Thermodynamic Systems
It is defined as a system that has been limited to fixed surroundings, either by hypothetical or real boundaries. This means all other quantities outside the system are considered to be part of the surroundings, which when added make the universe.
There are three thermodynamic systems:
- Isolated System that cannot exchange energy and mass with its surroundings, just like the universe.
- Closed System, where energy transfer takes place across the boundaries, but the same is not the case with the transfer of mass. The best example of a refrigerator.
- Open System, where both mass and energy undergo transfer between the system and its external area
Thermodynamic Processes
As the title itself states, it is a process where a change in a system is absorbed, specifically from one type of equilibrium micro-state to another.
Thermodynamic Equilibrium
Thermodynamic equilibrium is defined as a condition where the system does not absorb any change in property with time unless an external system acts on it.
Thermodynamic equilibrium can be clearly reflected in the fact that, at a given state, all properties in a system will exhibit fixed values. When one value of a property is altered, it affects the state of the entire system, changing it to another one. But, for a system that is assured of a state of equilibrium, the value of properties doesn’t undergo any change in case it is isolated from the surroundings.
For example, the temperature remains the same in the case of thermal equilibrium, mechanical equilibrium where there is no change in the pressure, chemical equilibrium where the composition does not alter with time, phase equilibrium, and so on.
What are the various branches of thermodynamics?
As we understand the laws of thermodynamics and before we embark on the properties of thermodynamics and its equilibrium, let us list out the branches or types of thermodynamics with respect to their study:
- Classical Thermodynamics
- Statistical Thermodynamics
- Chemical Thermodynamics
- Equilibrium Thermodynamics
Classical Thermodynamics: Under this, the matter’s behavior is studied at a macroscopic approach where temperature and pressure are considered for analyses. This branch is instrumental in measuring other properties and arriving at the behavioral aspects of the matter that are put under the process.
Statistical Thermodynamics: Where every molecule is under focus, understanding how they interact and learn their behavior in groups.
Chemical Thermodynamics: Correlating work and heat
Equilibrium Thermodynamics: Understanding energy and matter and their transformations while they attain the state of equilibrium.
Properties of Thermodynamics
The properties of thermodynamics represent its features in a system that have the potential to define and determine the state and condition of the system, both extensively and intensively.
While pressure and temperature are intensive in type, volume, enthalpy, and energy are extensive properties. The intensive kind of properties are those that are not altered by the matter and its size, while the extensive ones are characterized by the mass of the system.
In this aspect, let us understand these properties:
Enthalpy
Enthalpy is defined as the measure of energy in a thermodynamic system where its quantity is equal to the total heat present inside the system and is equivalent to the internal energy of the system combined with the product of volume and pressure.
In mathematical terms, enthalpy is H, the sum of the internal energy is E, the product of the pressure is P, and volume is V.
H = E + PV
Entropy
Entropy is referred to as a thermodynamic quantity whose value is determined by the system’s physical state or condition. In simple terminology, entropy as a thermodynamic function is applied in the measurement of disorder.
Conclusion
The above article helped you understand all about the four thermodynamics laws, as well as their terms in much more detail. If you are studying these chapters in Physics, you might have been aware that this subject is very tough, as it contains multiple complex chapters or topics. So, for people who are struggling in this subject, the online interactive classes offered by the Tutoroot platform at cost-effective prices might be useful.
Frequently Asked Questions
What is thermodynamics?
Thermodynamics is a specialized study of heat and temperature and their various dimensions and concepts. These aspects related to heat and temperature, under thermodynamics and the inter-conversion of heat and other forms of energy form very interesting and crucial study in physics. There are four laws of thermodynamics that determine and drive their quantities and explain their quantitative nature. The credit to coin the word, thermodynamics goes to William Thomson, who gave this name in 1749.
What is meant by Thermodynamic Processes?
Thermodynamic processes are those where a change in a system is absorbed, specifically from one type of equilibrium micro-state to another.
What is the Thermodynamic Equilibrium?
Thermodynamic equilibrium is defined as a condition where the system does not absorb any change in property with time unless an external system acts on it.
Thermodynamic equilibrium can be clearly reflected in the fact that, at a given state, all properties in a system will exhibit fixed values. When one value of a property is altered, if affects the state of the entire system, changing it to another one. But, for a system that is assured of a state of equilibrium, the value of properties don’t undergo any change in case it is isolated from the surroundings.
Define Laws of Thermodynamics
The Laws of Thermodynamics are those that help individuals define fundamental physical quantities such as temperature, entropy, and energy, which play a crucial role in characterizing thermodynamic systems at thermodynamic equilibrium. This helps in finding out how each of these quantities generally behaves under different types of circumstances or changes. It is important to understand various concepts or chapters in Physics, as they are directly derived or indirectly connected to the Laws of Thermodynamics.
State Zeroth Law of Thermodynamics
Zeroth’s Law of Thermodynamics explains that two bodies that are individually in equilibrium with a third body will also be in thermal equilibrium with each other.
State First Law of Thermodynamics?
The First Law of Thermodynamics states that energy can neither be destroyed nor created, it can only be converted from one form to another.
State Second Law of Thermodynamics
The Second Law of Thermodynamics suggests that the entropy in an isolated system will always increase. As the isolated systems have a high tendency to evolve towards thermal equilibrium, which in turn means that the state of entropy of the system will be at maximum. This is mainly because the entropy of the universe only increases.
State Third Law of Thermodynamics
the temperature of a system reaches absolute zero, then at the same time the entropy of a system will reach a constant value.
What is Entropy in Thermodynamics?
Entropy is defined as a measure of a system’s thermal energy per unit temperature, which is unavailable for doing useful work.
