What is Dipole Moment? – Formula, Example, Importance
The fascinating world of chemistry encompasses a multitude of concepts, each playing a crucial role in understanding the behavior of atoms and molecules. One such concept that holds immense significance is the dipole moment. In this blog post, we will delve into the depths of the dipole moment, uncovering its definition, formula, calculation methods, importance, and examples. Whether you’re a chemistry enthusiast or a student seeking clarification, this comprehensive guide will equip you with the knowledge to comprehend the intricacies of dipole moments.
What is a Dipole Moment?
A dipole moment is a measurement that quantifies the polarity of a molecular bond or an entire molecule. It signifies the separation of positive and negative charges within a molecule, indicating its overall polarity. The higher the dipole moment, the more polar the molecule.
To understand this concept better, let’s take the example of a water molecule. Water, with its formula H2O, is a polar molecule due to the difference in electronegativity between oxygen and hydrogen atoms. The oxygen atom has a higher electronegativity, causing the electrons to spend more time around it, giving it a partial negative charge (δ-). On the other hand, the hydrogen atoms, with lower electronegativity, acquire partial positive charges (δ+). This unequal distribution of charges creates a dipole moment in the water molecule, contributing to its unique properties such as hydrogen bonding and high surface tension.
The formula of Dipole Moment
The formula to calculate the dipole moment (μ) of a molecule is:
μ = Q × r
Here, Q represents the magnitude of the charge separation and r signifies the distance between the charges. The dipole moment is measured in Debye units (D), where 1 Debye = 3.336 × 10^−30 coulomb-meter.
How to Calculate Dipole Moment?
Calculating dipole moment involves understanding the concept of polarity in molecules. In simple terms, a dipole moment is a measure of the separation of positive and negative charges within a molecule. It’s like determining how much a molecule leans towards having a positive side and a negative side.
Here’s how you calculate dipole moment:
Steps to Calculate Dipole Moment
Identify Polar Molecules:
Start by identifying molecules that have polar bonds. Polar molecules have an uneven distribution of electrons, creating a positive and a negative end.
Determine Bond Polarity:
Look at the electronegativity values of atoms involved in the bond. Electronegativity is the tendency of an atom to attract electrons in a chemical bond. If there’s a significant difference in electronegativity between atoms in a bond, it’s likely polar.
Draw the Molecular Structure:
Visualize the structure of the molecule and determine its shape. Some molecules are symmetric, while others are asymmetrical, leading to differences in polarity.
Calculate the Dipole Moment:
Once you have a polar molecule, calculate its dipole moment using the formula:
Dipole Moment = Charge × Distance
Charge: Multiply the magnitude of the charges at either end of the molecule. If one end has a partial positive charge (+δ) and the other has a partial negative charge (-δ), multiply these values.
Distance: Measure the distance between the positive and negative ends of the molecule. This distance is usually given in picometers (pm) or meters (m).
Consider Vector Addition:
If the molecule has multiple polar bonds, consider vector addition to determine the overall dipole moment. This involves considering both the magnitude and direction of individual bond dipole moments.
Units
The dipole moment is typically measured in Debye (D) units. 1 Debye is equal to
3.336×10−30 Coulomb meters.
Example: Let’s take the water molecule (H2O) as an example. Oxygen (O) is more electronegative than hydrogen (H), so it attracts electrons more strongly.
- The water molecule has a bent shape, with the oxygen atom at the center and two hydrogen atoms at an angle.
- The oxygen end of the molecule has a partial negative charge, while the hydrogen ends have partial positive charges.
- Calculate the dipole moment by multiplying the charge at each end by the distance between them.
In summary, calculating dipole moment involves understanding the polarity of molecules, determining the charges and distances involved, and applying the dipole moment formula to quantify the separation of charges within the molecule.
Importance of Dipole Moment
The dipole moment plays a crucial role in various aspects of chemistry, serving as an invaluable tool for scientists and researchers. Here are some key reasons why the dipole moment holds significant importance:
- Polarity determination: The dipole moment helps ascertain the polarity of a molecule, which, in turn, impacts its chemical properties and interactions with other substances. Understanding molecular polarity is essential in fields like solubility, intermolecular forces, and biological processes.
- Predicting molecular behavior: By knowing the dipole moment, scientists can predict how molecules will interact with each other and their surroundings. This knowledge aids in understanding various chemical reactions, such as acid-base interactions, nucleophilic substitutions, and solvent effects.
- Designing efficient molecules: In fields like drug discovery and material science, dipole moment measurements guide researchers in designing molecules with desired properties. By manipulating the dipole moment, researchers can fine-tune factors like solubility, stability, and electronic properties to optimize their applications.
- Analyzing spectroscopic data: Dipole moment values are essential in the interpretation of spectroscopic techniques like infrared (IR) and microwave spectroscopy. The observed spectral data is often correlated with dipole moment values, enabling the identification and characterization of molecular compounds.
Examples of Dipole Moment
Let’s explore two examples to understand the concept of dipole moment more clearly:
Water molecule: A polar entity
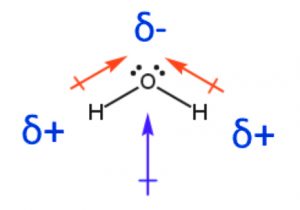
The water molecule (H2O) is renowned for its polarity, and the dipole moment helps elucidate this characteristic. Oxygen, with an electronegativity of 3.44, attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on oxygen. The two hydrogen atoms, with an electronegativity of 2.20, develop partial positive charges (δ+) due to the electron cloud’s shift towards oxygen. The dipole moment of water is approximately 1.85 Debye units, indicating a substantial charge separation and a polar nature. This polarity contributes to the unique properties of water, including its high boiling point, surface tension, and the ability to dissolve various substances.
Beryllium-fluorine compound: A nonpolar compound
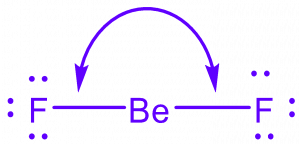
In contrast to water, the beryllium-fluorine (BeF2) compound exhibits a nonpolar nature. Beryllium, with an electronegativity of 1.57, shares its electrons equally with two fluorine atoms, each having an electronegativity of 3.98. Due to the symmetrical arrangement and balanced charges, the dipole moment of BeF2 is zero. This compound’s nonpolarity impacts its interactions with other substances and explains properties like non-conductivity and low boiling point.
Uses of Dipole Moment
The dipole moment finds practical applications in several domains, including:
- Chemical synthesis and analysis: Dipole moment values aid in identifying and characterizing various compounds, facilitating their synthesis and analysis in laboratories.
- Solvent selection: The polarity of a solvent determines its suitability for different chemical reactions. The knowledge of dipole moment helps researchers choose the appropriate solvent for desired reaction outcomes.
- Materials science: Dipole moments play a crucial role in material properties like stability, optical behavior, and conductivity. Researchers utilize this information to design and engineer materials with specific characteristics.
- Molecular modeling: In computational chemistry, dipole moment measurements serve as input parameters for molecular modeling simulations. This data enables researchers to predict molecular behavior and study complex systems.
Final Notes
Understanding the concept of dipole moment is essential in unraveling the behavior of atoms and molecules. It helps us comprehend the polarity of compounds, predict their interactions, and design substances with desired properties. By leveraging the formula, calculation methods, and examples provided in this blog post, you can navigate the world of dipole moments confidently. Remember, the dipole moment serves as a guiding force in the realm of chemistry, opening doors to countless research opportunities and providing a deeper understanding of the molecular world.
Keen on easily mastering intricate concepts, as demonstrated above? Dive into our Tutoroot Blog section for simplified learning. Enhance your comprehension of subjects and have your queries answered through Tutoroot’s online tuition. Discover the benefits of Tutoroot’s online home tuitions by scheduling a FREE DEMO session today.
FAQs
What is the direction of the dipole moment?
A: The direction of the dipole moment is from the positive to the negative end of the molecule.
Q: Dipole moment definition
A: Dipole moment is a measure of the separation of charge within a molecule, indicating the molecule’s polarity.
Q: What is dipole moment in chemistry?
A: In chemistry, dipole moment refers to the separation of charge within a molecule, leading to a partial positive and a partial negative end.
Q: What is the dipole moment of water?
A: The dipole moment of water is a result of the electronegativity difference between oxygen and hydrogen atoms, creating a partial positive and partial negative charge.
