What is the Tyndall Effect? – Examples, Process
The Tyndall Effect is one of the important concepts in chemistry, in this article we have a look at the Tyndall effect, Examples, and an in-depth explanation of its experiment procedure.
What is the Tyndall Effect?
The Tyndall Effect, named after the Irish physicist John Tyndall, refers to the scattering of light by colloidal particles suspended in a transparent medium. When a beam of light passes through a colloidal solution or a fine suspension, the light is scattered in all directions, making the path of the light visible. This phenomenon is observed when the size of the particles in the dispersed phase of the mixture is like the wavelength of light.
Examples of the Tyndall Effect
Here are the best examples of the Tyndall effect,
- When a flashlight is shone on a foggy night, the beam becomes visible due to the Tyndall Effect.
- Blue eyes appear blue due to the scattering of light in the iris, exhibiting the Tyndall Effect.
- Milk looks white because the fat and protein particles in it scatter light, demonstrating the Tyndall Effect.
Tyndall Effect Diagram
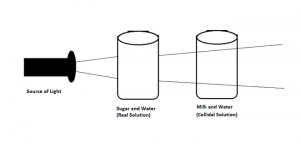
The diagram above illustrates the Tyndall Effect phenomenon. When a beam of light is directed through a colloidal solution, the particles present in the solution scatter the light, making the beam visible. This scattering of light gives rise to the characteristic cone of light observed in the Tyndall Effect.
Tyndall Effect Experiment
To observe the Tyndall Effect firsthand, you can conduct a simple experiment using readily available materials. Here’s how:
Materials Needed:
- Flashlight or laser pointer
- Clear glass or container
- Water
- Cornstarch or flour (optional, for creating a colloidal solution)
Procedure:
- Fill the clear glass or container with water.
- If desired, add a small amount of cornstarch or flour to the water and stir to create a colloidal solution.
- Dim the lights in the room.
- Shine the flashlight or laser pointer through the water-filled glass or container.
- Observe the beam of light passing through the water. You should be able to see the scattered light, indicating the presence of suspended particles in the water.
- Try shining the light through plain water too to compare the difference in the scattered light’s intensity.
Concept of Tyndall Effect in Colloidal Solution
The Tyndall Effect is more pronounced in colloidal solutions compared to true solutions or suspensions. In colloids, the dispersed phase consists of particles intermediate in size between true solutions and suspensions. These particles scatter light effectively, leading to the visibility of the beam passing through the mixture. This property is utilized in industries for analyzing colloidal solutions and in scientific research for studying particle size and distribution.
Final Verdict
The Tyndall Effect is a fascinating optical phenomenon that helps in characterizing colloids based on their ability to scatter light. Understanding this effect is crucial in various fields, from pharmaceuticals to environmental science, where the behavior of colloidal solutions plays a significant role.
In this article, we explore the experimental procedure and diagram for the Tyndall effect. To delve deeper into these concepts, visit our blog section. If you’re seeking personalized online tuition, Tutoroot offers excellent options. Our chemistry online tuition sessions are designed to clarify any doubts you may have about various concepts. Click here to book a FREE DEMO session.
FAQs
What is the Tyndall Effect?
The Tyndall Effect refers to the scattering of light by colloidal particles suspended in a transparent medium.
Who discovered the Tyndall Effect?
The Tyndall Effect is named after the Irish physicist John Tyndall, who first observed and explained this phenomenon.
Give some Tyndall Effect Examples
Examples of the Tyndall Effect include the visibility of a flashlight beam in fog, blue eyes appearing blue, and the white color of milk when light is passed through it.
