What is Valency Bond Theory? – Postulates of Valency Bond Theory
Introduction
In chemistry, understanding how atoms bond to form molecules is fundamental. One of the key theories explaining this process is the Valence Bond Theory (VBT). This theory provides insight into how atoms combine to form stable compounds by sharing electrons, leading to the formation of covalent bonds. The Valence Bond Theory helps in understanding the nature of bonds, the geometry of molecules, and the behavior of electrons in chemical bonding. In this article, we’ll explore the definition, postulates, history, and applications of Valency Bond Theory, along with the concept of hybridization.
What is the Valency Bond Theory?
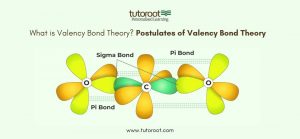
Valence Bond Theory (VBT) is a quantum mechanical theory that explains the formation of covalent bonds in molecules. The theory proposes that atomic orbitals overlap when atoms form bonds. When the atomic orbitals of two atoms overlap, the electrons in these orbitals pair up, leading to the formation of a covalent bond. This overlap can occur in different ways, such as head-to-head overlap (sigma bonds) or side-to-side overlap (pi bonds). The strength and stability of the bond depend on the extent of the overlap; greater overlap results in a stronger bond.
In VBT, the electrons that participate in bonding are considered to be confined to the space between the bonded atoms. The theory also explains that the geometry of a molecule is determined by the spatial arrangement of the orbitals involved in bonding. This spatial arrangement leads to the formation of specific bond angles and molecular shapes.
One of the essential aspects of VBT is the concept of hybridization, where atomic orbitals mix to form new hybrid orbitals that are more suitable for bonding. These hybrid orbitals have different shapes and energies than the original atomic orbitals, allowing atoms to form bonds that are energetically favorable and geometrically optimal.

Valence Bond Theory Postulates
Valence Bond Theory is based on several postulates that help explain how atoms bond to form molecules:
- Atomic Orbital Overlap: The primary postulate of VBT is that a covalent bond forms when atomic orbitals from two atoms overlap. This overlap allows the electrons in the orbitals to pair up, leading to bond formation. The extent of overlap determines the bond strength and stability.
- Formation of Sigma and Pi Bonds: VBT explains that bonds can be classified into sigma (σ) and pi (π) bonds based on how the atomic orbitals overlap. Sigma bonds are formed by the head-to-head overlap of orbitals, resulting in a bond that lies along the axis connecting the two nuclei. Pi bonds, on the other hand, are formed by the side-to-side overlap of orbitals, leading to a bond above and below the axis of the bonded atoms.
- Hybridization of Orbitals: To account for molecular geometry, VBT introduces the idea of orbital hybridization. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals more suitable for bonding. These hybrid orbitals have different shapes and energies, allowing for the formation of bonds with specific angles and molecular shapes.
- Electron Pairing: VBT assumes that electrons in a covalent bond are paired. This pairing is due to the overlapping of atomic orbitals, where each electron from the bonding atoms pairs up with an electron from another atom. The paired electrons are situated between the two nuclei, leading to the creation of a stable covalent bond.
- Localized Electrons: VBT suggests that bonding electrons are confined to the region between the atoms involved. This means the electron density is concentrated in the region between the two nuclei, leading to a covalent bond with specific bond length and energy.
- Energy Considerations: VBT suggests that forming a covalent bond is energetically advantageous. The overlap of atomic orbitals results in a lower potential energy for the bonded atoms, leading to the formation of a stable molecule.
History of Valency Bond Theory
Valence Bond Theory was first proposed by Walter Heitler and Fritz London in 1927. They applied quantum mechanics to explain how two hydrogen atoms bond to form a hydrogen molecule (H2). Their work laid the foundation for the development of VBT, which was later expanded and refined by chemists such as Linus Pauling and John C. Slater.
Linus Pauling, in particular, played a significant role in popularizing and expanding VBT. He introduced the concept of hybridization in 1931, which helped explain the geometry of molecules and the formation of bonds in more complex molecules. Pauling’s work on VBT was instrumental in the development of the modern understanding of chemical bonding and molecular structure.
The historical development of VBT was marked by the integration of quantum mechanics into chemistry. This integration allowed chemists to explain the nature of chemical bonds and the behavior of electrons in molecules more accurately. VBT became a cornerstone of chemical bonding theory, providing a framework for understanding how atoms bond to form molecules.
Despite its success, VBT faced competition from the Molecular Orbital Theory (MOT), which provided an alternative explanation for chemical bonding. However, VBT remains an essential tool for understanding the bonding in many molecules, especially those with localized electron pairs and well-defined molecular geometries.
Types of Hybridization
Hybridization is a crucial concept in VBT that explains the formation of bonds in molecules with specific geometries. There are several types of hybridization, each resulting in different molecular shapes and bond angles:
- sp Hybridization: This hybridization occurs when one s orbital combines with one p orbital to create two sp hybrid orbitals. The sp hybrid orbitals are linearly arranged with a bond angle of 180°, resulting in a linear molecular geometry. An example of sp hybridization is the bonding in beryllium chloride (BeCl2).
- sp2 Hybridization: In this type, one s orbital merges with two p orbitals, resulting in three sp2 hybrid orbitals. These orbitals are arranged in a trigonal planar geometry with a bond angle of 120°. An example of sp2 hybridization is the bonding in boron trifluoride (BF3).
- sp3 Hybridization: sp3 hybridization occurs when one s orbital and three p orbitals mix to form four sp3 hybrid orbitals. These orbitals are arranged in a tetrahedral geometry with a bond angle of 109.5°. An example of sp3 hybridization is the bonding in methane (CH4).
- sp3d Hybridization: This type of hybridization involves the mixing of one s orbital, three p orbitals, and one d orbital to form five sp3d hybrid orbitals. The resulting geometry is trigonal bipyramidal, with bond angles of 90° and 120°. An example of sp3d hybridization is the bonding in phosphorus pentachloride (PCl5).
- sp3d2 Hybridization: In sp3d2 hybridization, one s orbital, three p orbitals, and two d orbitals mix to form six sp3d2 hybrid orbitals. The resulting geometry is octahedral, with bond angles of 90°. An example of sp3d2 hybridization is the bonding in sulfur hexafluoride (SF6).
Applications of Valency Bond Theory
Valence Bond Theory has various applications in understanding chemical bonding, molecular geometry, and the reactivity of molecules. It is used to predict the shapes of molecules, the bond angles, and the type of bonds (sigma or pi) formed between atoms. VBT also helps in understanding the behavior of electrons in reactions, the formation of complex ions, and the stability of molecules.
Limitations of Valency Bond Theory
While Valence Bond Theory is useful, it has some limitations. It does not explain the magnetic properties of molecules or the color of compounds. VBT also struggles to describe the bonding in molecules with delocalized electrons, such as benzene. Additionally, it cannot explain the bonding in transition metal complexes accurately, where Molecular Orbital Theory (MOT) is more effective.
Final Notes
Valence Bond Theory provides a foundational understanding of how atoms bond to form molecules. By explaining the overlap of atomic orbitals and the concept of hybridization, VBT offers insights into molecular geometry, bond strength, and the behavior of electrons in chemical bonding. Despite its limitations, VBT remains a valuable tool for chemists and students alike in understanding the nature of chemical bonds.
If you’re looking for more simplified explanations like the ones above, visit the Tutoroot Blog for a wealth of learning resources. Enhance your understanding with Tutoroot’s expert Chemistry online Tuition. Ready to excel in your studies? Schedule a FREE DEMO session with Tutoroot’s online home tuition and experience personalized learning tailored to your needs.
FAQs
What is VBT theory?
VBT theory, or Valence Bond Theory, is a quantum mechanical theory that explains how atoms bond to form molecules by overlapping their atomic orbitals.
What are the postulates of Valence Bond Theory?
The postulates of Valence Bond Theory include atomic orbital overlap, the formation of sigma and pi bonds, hybridization of orbitals, electron pairing, localized electrons, and energy considerations.
Define Orbital Hybridization.
Orbital hybridization is the process of mixing atomic orbitals to form new hybrid orbitals that are more suitable for bonding. This process helps explain the geometry of molecules and the bond angles.
Explain Valence Bond Theory with an example.
Valence Bond Theory can be explained with the example of the hydrogen molecule (H2). According to VBT, the bond forms due to the overlap of the 1s orbitals from two hydrogen atoms, resulting in a sigma bond that holds the atoms together.