What are Amines? – Types of Amines, Properties
Introduction
Amines are a crucial class of organic compounds in chemistry, characterized by their nitrogen-containing functional group. They are compounds derived from ammonia (NH₃) where one or more hydrogen atoms are substituted with alkyl or aryl groups. Amines play a big role in various biological processes and are widely used in the chemical industry for pharmaceuticals, dyes, and polymers. Understanding the structure, types, and properties of amines is essential for students studying organic chemistry. This article will thoroughly explore amines, including their formula, examples, structure, types, preparation, uses, and properties.
What are Amines?
Amines are organic compounds that feature a nitrogen atom bonded to one or more alkyl or aryl groups. The simplest amine, ammonia (NH₃), can be seen as the parent compound, where the nitrogen atom is bonded to three hydrogen atoms. When one or more of these hydrogen atoms are replaced by organic groups, the resulting compound is known as an amine.
Amines can be classified into primary, secondary, and tertiary amines based on the number of organic groups attached to the nitrogen atom. They are also categorized into aliphatic and aromatic amines, depending on whether the nitrogen is attached to an alkyl or aryl group. Amines exhibit basic properties because the nitrogen atom possesses a lone pair of electrons that can attract and accept a proton. This basicity plays a crucial role in the reactivity and applications of amines in various chemical reactions.
Amines are ubiquitous in nature and are found in various biological molecules, including neurotransmitters and amino acids. Their importance extends beyond biology, as they are used extensively in the synthesis of pharmaceuticals, agrochemicals, and polymers. Understanding the chemistry of amines is essential for students and professionals in organic chemistry.

Amines Formula
The general formula for amines depends on their classification:
- Primary Amines: The general formula is RNH₂, where R represents an alkyl or aryl group.
- Secondary Amines: The general formula is R₂NH, where R represents two alkyl or aryl groups.
- Tertiary Amines: The general formula is R₃N, where R represents three alkyl or aryl groups.
In these formulas, the nitrogen atom is bonded to one or more organic groups and retains a lone pair of electrons, which contributes to the basicity of amines.
Amines Examples
Amines can be found in various forms, ranging from simple molecules to complex biological structures. Some common examples include:
- Methylamine (CH₃NH₂): A simple primary amine utilized in manufacturing pesticides and solvents.
- Dimethylamine ((CH₃)₂NH): A secondary amine used in the manufacture of pharmaceuticals and rubber chemicals.
- Trimethylamine ((CH₃)₃N): A tertiary amine known for its fishy odor, used in the production of cleaning agents and surfactants.
- Aniline (C₆H₅NH₂): An aromatic amine used in the production of dyes and pigments.
- Epinephrine (Adrenaline): A biologically significant amine that acts as a neurotransmitter and hormone.
These examples highlight the diversity of amines, ranging from simple alkylamines to complex aromatic amines, each with unique applications and properties.
Structure of Amines
The structure of amines is characterized by a nitrogen atom bonded to one or more organic groups. The nitrogen atom has a lone pair of electrons, which plays a crucial role in the basicity and reactivity of amines. The structure of amines can be understood in terms of their hybridization and geometry.
In primary amines, the nitrogen atom is sp³ hybridized, leading to a tetrahedral geometry around the nitrogen. The three hydrogen atoms (or alkyl groups in secondary and tertiary amines) and the lone pair of electrons occupy these positions, resulting in a pyramidal shape. The bond angles in amines are slightly less than the ideal tetrahedral angle of 109.5° due to the repulsion between the lone pair and the bonding pairs of electrons.
Secondary and tertiary amines have similar structures, with the nitrogen atom bonded to two or three alkyl or aryl groups, respectively. The presence of larger alkyl groups can lead to steric hindrance, affecting the reactivity and physical properties of the amines. In aromatic amines, such as aniline, the nitrogen atom is bonded to an aryl group, and the lone pair of electrons can participate in resonance with the aromatic ring, influencing the electronic properties of the amine.
The structure of amines also influences their physical properties, such as boiling point and solubility. For example, primary amines have higher boiling points than secondary and tertiary amines due to hydrogen bonding. Understanding the structure of amines is essential for predicting their behavior in chemical reactions and their interactions with other molecules.
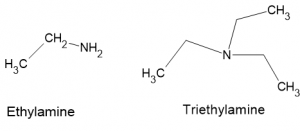
Types of Amines
Amines can be classified into different types based on the number of organic groups attached to the nitrogen atom:
- Primary Amines: In primary amines, the nitrogen atom is connected to one alkyl or aryl group along with two hydrogen atoms. Examples include methylamine (CH₃NH₂) and ethylamine (C₂H₅NH₂). Primary amines are basic and can participate in various chemical reactions, such as nucleophilic substitution and condensation reactions.
- Secondary Amines: In secondary amines, the nitrogen atom is attached to two alkyl or aryl groups and one hydrogen atom. Examples include dimethylamine ((CH₃)₂NH) and diethylamine ((C₂H₅)₂NH). Secondary amines are also basic and can form hydrogen bonds, but their boiling points are lower than those of primary amines due to reduced hydrogen bonding.
- Tertiary Amines: Tertiary amines have nitrogen atoms bonded to three alkyl or aryl groups and no hydrogen atoms. Examples include trimethylamine ((CH₃)₃N) and triethylamine ((C₂H₅)₃N). Tertiary amines are less basic than primary and secondary amines and do not participate in hydrogen bonding, leading to even lower boiling points.
- Aromatic Amines: Aromatic amines have the nitrogen atom bonded to an aromatic ring, such as in aniline (C₆H₅NH₂). The nitrogen atom’s lone pair of electrons can engage in resonance with the aromatic ring, influencing both the reactivity and basicity of the amine. Aromatic amines are commonly used in creating dyes, pigments, and pharmaceuticals.
Each type of amine has unique properties and applications, making them valuable in various fields of chemistry and industry.
Preparation of Amines
Amines can be prepared through various methods, depending on the type and desired properties of the amine. Some common methods include:
- Reduction of Nitriles: Nitriles can be reduced to primary amines using hydrogen gas and a metal catalyst or reducing agents like lithium aluminum hydride (LiAlH₄).
- Reduction of Nitro Compounds: Aromatic nitro compounds can be reduced to aromatic amines using reducing agents such as tin and hydrochloric acid or catalytic hydrogenation.
- Alkylation of Ammonia: Primary, secondary, and tertiary amines can be synthesized by the alkylation of ammonia or amines with alkyl halides. This reaction requires a suitable base to neutralize the acidic by-products.
- Gabriel Phthalimide Synthesis: This method is used to prepare primary amines from phthalimide, which is treated with a strong base, followed by alkylation and hydrolysis.
Each method has its advantages and is chosen based on the desired amine and its applications.
Amines Uses
Amines have a wide range of applications in various industries, including:
- Pharmaceuticals: Amines are used in the synthesis of drugs and active pharmaceutical ingredients (APIs). Many antibiotics, analgesics, and antihistamines contain amine groups.
- Agriculture: Amines are used in pesticides, herbicides, and fertilizer production to enhance crop yield and protect plants from pests.
- Dyes and Pigments: Aromatic amines, such as aniline, are used in making dyes and pigments for textiles, plastics, and inks.
- Polymers: Amines are used in polymer production, such as polyurethane, and as curing agents for epoxy resins.
Properties of Amines
Amines possess several unique properties:
- Basicity: Amines are basic due to the presence of a lone pair of electrons on the nitrogen atom. They can accept protons to form ammonium ions.
- Hydrogen Bonding: Primary and secondary amines can form hydrogen bonds, leading to higher boiling points and solubility in water compared to tertiary amines.
- Reactivity: Amines are nucleophilic and can participate in various chemical reactions, including alkylation, acylation, and condensation reactions.
Final Notes:
If you’re looking to deepen your understanding of similar topics, the Tutoroot Blog is a great place to start. For more personalized guidance, Tutoroot’s chemistry online tuitions are here to help you excel. Don’t miss the opportunity to experience Tutoroot’s online home tuitions—schedule your FREE DEMO session today and take the first step toward mastering your subject.
FAQs
Write Amines Formula
The general formula for amines depends on their type: RNH₂ for primary amines, R₂NH for secondary amines, and R₃N for tertiary amines.
Give some Amines Examples
Examples include methylamine (CH₃NH₂), dimethylamine ((CH₃)₂NH), trimethylamine ((CH₃)₃N), and aniline (C₆H₅NH₂).
What are the Types of Amines?
Amines are classified into primary, secondary, tertiary, and aromatic amines based on the number of organic groups attached to the nitrogen atom.
Write a few Amines Uses
Amines are used in pharmaceuticals, agriculture, dyes and pigments, and polymer production.

