What Sets Endothermic and Exothermic Reactions Apart?
Introduction
In chemistry, reactions can either absorb or release energy. Understanding the difference between endothermic and exothermic reactions is crucial for grasping how energy changes affect chemical processes. These reactions are fundamental concepts in thermodynamics, playing a key role in various natural and industrial processes. This article will explore the definitions, examples, and differences between endothermic and exothermic reactions, making it clear and understandable for students.
What are Endothermic Reactions?
Definition and Explanation
Endothermic reactions are chemical reactions that absorb energy from their surroundings in heat. The term “endothermic” is derived from the Greek words “endo,” meaning inside, and “therme,” meaning heat. In an endothermic reaction, the energy needed to break the bonds of the reactants exceeds the energy released when new bonds are formed in the products. As a result, there is a net intake of energy, causing the surrounding environment to cool down.
These reactions are characterized by a positive enthalpy change (ΔH > 0), indicating that the energy of the products is higher than that of the reactants. Endothermic reactions are essential in various natural processes, such as photosynthesis in plants, where sunlight is absorbed to convert carbon dioxide and water into glucose and oxygen.
In a laboratory setting, endothermic reactions often require continuous heating to proceed, as they rely on an external energy source to overcome the activation energy barrier. These reactions are not spontaneous and require careful control of temperature and energy input.
Endothermic Reaction Examples
- Photosynthesis:
- Equation: 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
- Description: Plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen.
2. Melting of Ice:
- Equation: H₂O(s) + heat → H₂O(l)
- Description: Ice absorbs heat energy to transition from solid to liquid.
3. Evaporation of Water:
- Equation: H₂O(l) + heat → H₂O(g)
- Description: Water absorbs heat to change from liquid to gas.
4. Thermal Decomposition of Calcium Carbonate:
- Equation: CaCO₃(s) + heat → CaO(s) + CO₂(g)
- Description: Calcium carbonate decomposes into calcium oxide and carbon dioxide upon heating.
5. Dissolving Ammonium Nitrate in Water:
- Equation: NH₄NO₃(s) + heat → NH₄⁺(aq) + NO₃⁻(aq)
- Description: Ammonium nitrate absorbs heat when dissolved in water, causing a cooling effect.
What are Exothermic Reactions?
Definition and Explanation
Exothermic reactions are chemical reactions that release energy into their surroundings, typically in the form of heat. The word “exothermic” originates from the Greek words “exo,” meaning “outside,” and “therme,” meaning “heat.” In an exothermic reaction, the energy released during the formation of bonds in the products is greater than the energy required to break the bonds in the reactants. This results in a net release of energy, causing the surrounding environment to heat up.
Exothermic reactions have a negative enthalpy change (ΔH < 0), indicating that the energy of the products is lower than that of the reactants. These reactions are often spontaneous and can occur rapidly, sometimes producing light, heat, or sound as by-products.
Exothermic reactions are common in everyday life and are essential in various industrial processes, such as combustion in engines and the setting of concrete. They play a vital role in biological systems as well, including cellular respiration, where glucose is broken down to release energy for cellular activities.
Exothermic Reaction Examples
- Combustion of Methane:
- Equation: CH₄ + 2O₂ → CO₂ + 2H₂O + energy
- Description: Methane burns in oxygen, releasing energy in the form of heat and light.
2. Respiration:
- Equation: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy
- Description: Glucose is oxidized in cells to release energy, carbon dioxide, and water.
3. Neutralization of Hydrochloric Acid and Sodium Hydroxide:
- Equation: HCl + NaOH → NaCl + H₂O + energy
- Description: When hydrochloric acid and sodium hydroxide react, they release heat.
4. Formation of Ice:
- Equation: H₂O(l) → H₂O(s) + energy
- Description: Water releases energy when it freezes, turning into ice.
5. Rusting of Iron:
- Equation: 4Fe + 3O₂ → 2Fe₂O₃ + energy
- Description: Iron reacts with oxygen to form rust, releasing energy in the process.
Difference Between Endothermic Reactions and Exothermic Reactions
Endothermic and exothermic reactions differ primarily in how they handle energy. Endothermic reactions draw energy from their surroundings, causing a reduction in the temperature near the reaction site. In contrast, exothermic reactions release energy, causing an increase in the surrounding temperature. These reactions can be identified by measuring the temperature change or by examining the enthalpy change (ΔH) associated with the reaction.
Understanding these differences is crucial in predicting how a reaction will behave under different conditions. For example, in industrial applications, controlling the temperature of an exothermic reaction is essential to prevent overheating, while ensuring a constant energy supply is vital for maintaining an endothermic reaction.
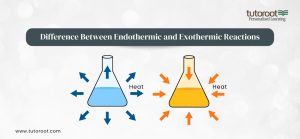
Endothermic and Exothermic Reactions Difference
| Property | Endothermic Reactions | Exothermic Reactions |
| Energy Flow | Absorbs energy from surroundings | Releases energy to surroundings |
| Temperature Change | Surrounding temperature decreases | Surrounding temperature increases |
| Enthalpy Change (ΔH) | Positive (ΔH > 0) | Negative (ΔH < 0) |
| Examples | Photosynthesis, Melting of Ice | Combustion of Methane, Respiration |
| Spontaneity | Non-spontaneous | Often spontaneous |
| Bond Formation | Energy required to break bonds > Energy released forming bonds | Energy released forming bonds > Energy required to break bonds |
| Common in | Endothermic reactions are common in processes that require energy input like cooking or electrolysis. | Exothermic reactions are found in processes that release energy like burning fuels or neutralization reactions. |
| Heat as a Reactant/Product | Heat is a reactant | Heat is a product |
| Industrial Examples | Electrolysis, Cooking | Combustion engines, Concrete setting |
| Biological Examples | Photosynthesis in plants | Respiration in animals |
Final Notes
Understanding the difference between endothermic and exothermic reactions is fundamental to grasping how chemical processes interact with energy. These reactions are not just theoretical concepts but are integral to everyday life and industrial processes. By recognizing whether a reaction absorbs or releases energy, scientists and engineers can control and optimize these reactions for various applications.
If you’re looking to deepen your understanding of subjects just like this one, the Tutoroot Blog offers more simplified and insightful explanations. For a personalized learning experience, consider exploring Tutoroot’s Chemistry Online Tuitions. Our expert tutors are here to help you master complex topics with ease. Start your journey with Tutoroot today by booking a FREE DEMO session and take the next step in your academic success.
FAQs
Define endothermic reaction
An endothermic reaction is a type of chemical reaction that absorbs energy from its environment, leading to a decrease in temperature.
What is exothermic reaction
An exothermic reaction is a chemical reaction that releases energy into its surroundings, causing an increase in temperature.
Give 10 examples of endothermic reactions with equations
- Photosynthesis: 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
- Melting of Ice: H₂O(s) + heat → H₂O(l)
- Evaporation of Water: H₂O(l) + heat → H₂O(g)
- Thermal Decomposition of Calcium Carbonate: CaCO₃(s) + heat → CaO(s) + CO₂(g)
- Dissolving Ammonium Nitrate in Water: NH₄NO₃(s) + heat → NH₄⁺(aq) + NO₃⁻(aq)
- Decomposition of Water: 2H₂O(l) + energy → 2H₂(g) + O₂(g)
- Baking: 2NaHCO₃(s) + heat → Na₂CO₃(s) + CO₂(g) + H₂O(g)
- Photosynthesis in Algae: 6CO₂ + 6H₂O + light energy → C₆H₁₂O₆ + 6O₂
- Electrolysis of Water: 2H₂O(l) + energy → 2H₂(g) + O₂(g)
- Cooking an Egg: Proteins in eggs absorb heat, changing their structure.
Write 5 differences between endothermic and exothermic reactions
- Endothermic reactions absorb energy, while exothermic reactions release energy.
- Endothermic reactions result in a temperature decrease, whereas exothermic reactions cause a temperature increase.
- Endothermic reactions have a positive enthalpy change (ΔH > 0), while exothermic reactions have a negative enthalpy change (ΔH < 0).
- In endothermic reactions, energy appears on the reactant side of the equation, whereas in exothermic reactions, it is found on the product side.
- Endothermic reactions often involve breaking bonds, while exothermic reactions involve bond formation.
