What is Exothermic Reaction? – Diagram, Reaction, Example
Introduction to Exothermic Reactions
In the fascinating realm of chemistry, we come across ax diverse array of reactions that occur all around us. These reactions involve the transformation of substances, releasing energy in various forms. One such type of reaction is the exothermic reaction. These reactions are particularly intriguing as they not only result in the formation of new substances but also give off heat to the surroundings. Let’s delve into the world of exothermic reactions, explore some examples, understand the underlying formula, dive into the process, and even take a closer look at the energy level diagram that depicts the energy changes during these reactions.
What is an Exothermic Reaction?
Before we delve into the intricacies of exothermic reactions, let’s grasp the basic concept. An exothermic reaction refers to a chemical or physical process that releases energy in the form of heat. In simpler terms, it’s like a reaction that shares its warmth with the environment. If you’ve ever held a hand warmer or felt the warmth emanating from a chemical reaction, you’ve experienced the effects of an exothermic reaction firsthand.
Exothermic Reaction Example
To better understand exothermic reactions, let’s consider some examples. One classic illustration is the combustion of fuel, such as when we burn wood in a fireplace. This combustion reaction results in the release of energy in the form of heat and light. The chemical equation for this exothermic reaction can be represented as:
\(C_{6} H_{12} O_{6}+6 O_{2} \rightarrow 6C O_{2} +6 H_{2}O+Energy\)
In this reaction, glucose (C6H12O6) and oxygen (O2) combine to form carbon dioxide (CO2) and water (H2O), while liberating a substantial amount of energy.
Another noteworthy example is the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) to form salt (NaCl) and water (H2O). The equation for this exothermic reaction can be depicted as:
\(HCl+NaOH \rightarrow NaCl+ H_{2} O+Energy\)
These examples highlight the exothermic nature of reactions and provide a glimpse into the diverse scenarios in which they occur.
Exothermic Reaction Formula
To understand the fundamental concepts governing exothermic reactions, let’s explore the underlying formula. In general, an exothermic reaction can be represented as:
Reactants -> Products + Energy
The energy term on the right side of the equation signifies the energy released during the reaction. It is crucial to note that the energy released in exothermic reactions is negative, denoting that it flows out from the system into the surroundings.
Process of Exothermic Reaction
Now that we comprehend the concept and have examined examples, let’s dive deeper into the process of an exothermic reaction. The journey of an exothermic reaction can be divided into several steps:
- Activation Energy: Every reaction requires a certain amount of energy, known as activation energy, to initiate the reaction. This energy kick-starts the breaking and making of chemical bonds.
- Bond Breaking: Once the activation energy is supplied, the reactant molecules start to break their existing bonds. This process requires energy input because bonds need to be broken to rearrange atoms and form new molecules.
- Bond Formation: As the reactant molecules break apart, new chemical bonds start to form between atoms, leading to the creation of different molecules. This step releases surplus energy.
- Energy Release: The energy released during bond formation ultimately takes the form of heat, manifesting as an increase in temperature in the surrounding environment.
- Product Formation: Lastly, after the energy release, the newly formed products stabilize and are ready to be observed.
Energy Level Diagram of Exothermic Reaction
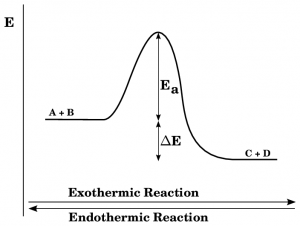
To visually depict the energy changes during an exothermic reaction, we can rely on an energy level diagram. This diagram showcases the energy of the reactants and products along with the activation energy required for the reaction. Let’s explore the key elements of this diagram:
- Reactant Energy: The energy of the reactants is represented on the left side of the diagram. It illustrates the initial energy state of the reactant molecules before the reaction occurs.
- Activation Energy: The energy hurdle that needs to be overcome for the reaction to commence is known as activation energy. It is depicted as a peak on the diagram.
- Product Energy: The energy of the products is shown on the right side of the diagram. It indicates the final energy configuration of the molecules involved in the reaction.
The diagram clearly depicts a decrease in energy from reactants to products, signifying the energy being released during the exothermic reaction.
Final Notes
Exothermic reactions play a vital role in countless chemical processes and natural phenomena. Understanding the concept behind these reactions, exploring examples, and visualizing the energy level diagram enhance our comprehension of the intricate world of chemistry. Embrace the captivating nature of exothermic reactions and let their warmth create a spark of curiosity in your mind. Remember, the world of chemistry is brimming with exciting discoveries waiting to be unraveled.
Explore the intricacies of the Exothermic reaction in our comprehensive article, providing insights into its process and examples. For those seeking simplified explanations of intricate concepts, we invite you to peruse our blog section. If you’re in search of premier online home tutoring, Tutoroot stands out as the optimal choice. Our seasoned faculty excels in elucidating complex subjects with ease. Feel free to click here and schedule a FREE DEMO to experience the difference!
FAQ’s
Examples of exothermic reactions:
There are numerous examples of exothermic reactions. Some common ones include:
- Combustion reactions: Burning wood, candles, or even gasoline releases energy.
- Neutralization reactions: The reaction between acids and bases to form water and salts often produces heat.
- Chemical explosions: Reactions in fireworks or explosives are exothermic, generating light and heat.
Exothermic reaction definition:
An exothermic reaction is a type of chemical reaction in which energy is released to the surroundings in the form of heat or light. This release of energy makes the surroundings warmer, and it’s a characteristic feature of reactions like combustion, respiration, and many everyday processes.
Why is respiration considered an exothermic reaction?
Respiration involves the breakdown of food molecules to release energy, making it an exothermic reaction. The chemical equation for cellular respiration is
\(C_{6} H_{12} O_{6}+6 O_{2} \rightarrow 6C O_{2} +6 H_{2}O+Energy\)
During this process, glucose (C₆H₁₂O₆) reacts with oxygen (O₂) to produce carbon dioxide (CO₂), water (H₂O), and energy.
