What are Endothermic Reactions? – Example, Reaction, Diagram
Introduction to Endothermic Reactions
Have you ever wondered how some chemical reactions can make things feel cold or require heat to get started? These are known as endothermic reactions, and they are the opposite of those reactions that give off heat. In this article, we will dive into the fascinating world of endothermic reactions, explaining what they are, and providing examples, equations, and even energy level diagrams to help you understand them better. So, let’s embark on this chemical journey and explore the world of endothermic reactions together!
What is an Endothermic Reaction?
An endothermic reaction is a chemical process in which energy is absorbed from the surroundings, usually in the form of heat. In simpler terms, it’s like a reaction that sucks up warmth from its environment. Instead of feeling the heat, you’d feel a drop in temperature because the reaction is stealing the heat energy.
Endothermic reactions are the opposite of exothermic reactions, where energy is released in the form of heat, making things feel hot. These reactions are quite common and play a significant role in various everyday processes and scientific experiments.
Endothermic Reaction Example
Let’s take a closer look at a common endothermic reaction example: the dissolution of ammonium nitrate in water. The chemical equation for this reaction is:
\(NH_{4} NO_{3} (s)+ H_{2}O \mapsto NH^{+} _{4} (aq)+ NO^{-} _{3}(aq)\)
In this reaction, solid ammonium nitrate (NH4NO3) is dissolved in water (H2O), forming aqueous ammonium ions (NH4+) and nitrate ions (NO3-). The reaction absorbs heat from its surroundings, making the solution colder.
Imagine holding a beaker of water and stirring in solid ammonium nitrate. As the reaction occurs, the beaker would start feeling cold to the touch because the energy required for the reaction is being taken from the water itself.
Endothermic Reaction Formula
Endothermic reactions are not limited to specific equations but share a common trait: they absorb energy. The general formula to represent an endothermic reaction is:
Reactants + Heat energy ⟶ Products
In this formula, “Reactants” are the substances that react together, and “Products” are the new substances formed after the reaction. The “Heat energy” is absorbed during the reaction, causing a drop in temperature.
Process of an Endothermic Reaction
Understanding the process of an endothermic reaction can be enlightening. Let’s break it down into a few key steps:
- Activation Energy: Every reaction, whether endothermic or exothermic, requires a certain amount of energy to get started. This initial energy is called “activation energy.” In the case of an endothermic reaction, the activation energy is relatively high.
- Reactant Collision: The reactants (the substances that will react) collide with each other. These collisions have to be energetic enough to overcome the activation energy barrier. When successful collisions occur, the reaction begins.
- Energy Absorption: During the reaction, the reactants absorb heat energy from their surroundings. This absorption of energy is what characterizes the reaction as endothermic.
- Product Formation: As the reaction progresses, new products are formed. These products are often at a lower energy state than the reactants, which is why the energy needs to be absorbed.
- Heat Removal: To prevent the reaction from overheating, the excess heat is usually removed from the surroundings, resulting in a drop in temperature.
A classic example of an endothermic process is photosynthesis, which brings us to our next question:
Why is Photosynthesis Considered an Endothermic Reaction?
Photosynthesis is a crucial process that takes place in plants, and it’s endothermic because it absorbs energy from the environment. During the process of photosynthesis, plants take in carbon dioxide (CO2) from the air and water (H2O) from the soil. With the help of sunlight, they convert these reactants into glucose (C6H12O6) and oxygen (O2).
The chemical equation for photosynthesis is:
6 CO2 (g) + 6 H2O (l) + energy (sunlight) → C6H12O6 (aq) + 6 O2 (g)
Here’s how photosynthesis is an endothermic reaction:
- Energy from the Sun: Photosynthesis relies on the energy from sunlight. This energy is required to initiate the reaction, overcoming the activation energy.
- Absorption of Carbon Dioxide and Water: Plants absorb carbon dioxide from the air and water from the soil. These reactants provide the building blocks for glucose production.
- Energy Storage: The energy from the sun is used to create glucose, which stores the energy for future use.
- Release of Oxygen: One of the products of photosynthesis is oxygen, which is released into the atmosphere.
- Temperature Drop: As the plant absorbs energy from the sun and converts it into glucose, there is a temperature drop in the surroundings, making photosynthesis an endothermic reaction.
Energy Level Diagram of an Endothermic Reaction
To truly grasp the concept of endothermic reactions, it helps to visualize them. Energy level diagrams provide a clear picture of how energy changes throughout a reaction. Let’s take a look at a typical energy level diagram for an endothermic reaction.
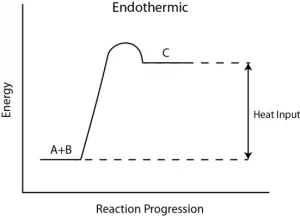
In this diagram:
- The horizontal axis represents the progression of the reaction from left (reactants) to right (products).
- The vertical axis represents the energy content.
Key points on the energy level diagram:
- The initial energy of the reactants is lower than the activation energy required for the reaction to occur. Therefore, the reactants need an external source of energy (heat, light, etc.) to overcome this energy barrier.
- As the reaction proceeds, the energy increases because heat energy is absorbed during the process.
- The energy of the products is higher than that of the reactants. This means that the products contain more energy than the reactants, which is why energy is absorbed from the surroundings.
Understanding this diagram helps illustrate why endothermic reactions feel cold. They start with lower-energy reactants, absorb energy during the reaction, and end with higher-energy products.
Endothermic Reaction Summary
In summary, endothermic reactions are essential chemical processes that absorb energy from their surroundings, causing a drop in temperature. They are characterized by high activation energy, energy absorption, and the formation of products with higher energy levels than the reactants.
Endothermic reactions are not only fascinating to learn about but also have many practical applications, from photosynthesis in plants to cold packs used to relieve injuries. By understanding these reactions, we can appreciate the complex world of chemistry that surrounds us and how it impacts our daily lives.
Final Notes
Now that you’ve explored the world of endothermic reactions, you have a deeper understanding of why some reactions make things feel cold and require an input of energy. Chemistry is full of exciting discoveries, and endothermic reactions are just one small part of this incredible science.
Dive into the fascinating realm of the Endothermic reaction with our in-depth article that guides you through its energy level diagrams and process. If you’re seeking a simplified understanding of complex concepts, make sure to explore our blog section. If you’re on the lookout for the finest online home tutoring experience, look no further than Tutoroot. Our experienced faculty specializes in making intricate concepts easy to grasp. Ready to take the first step? Click here to book a FREE DEMO now!
FAQ’s
Define Endothermic Reaction
An endothermic reaction is a chemical process that absorbs heat energy from its surroundings, making the environment feel colder. These reactions have a high activation energy and are characterized by energy absorption during the reaction.
What is the Endothermic Reaction Equation?
Endothermic reactions don’t have a specific equation but follow a general formula: Reactants + Heat energy ⟶ Products. The exact equation depends on the specific reaction under consideration.
Why is Photosynthesis Considered an Endothermic Reaction?
Photosynthesis is an endothermic reaction because it absorbs energy from the sun to convert carbon dioxide and water into glucose and oxygen. The energy from the sun is needed to overcome the activation energy, and the reaction results in a drop in temperature in the surroundings.
