What are Anode and Cathode? – Anode and Cathode Difference
Introduction
In the world of chemistry, understanding the concepts of anode and cathode is fundamental, especially when studying electrochemical reactions and electrolysis. These terms are pivotal in the functioning of batteries, electroplating, and other industrial processes. This article will delve into what anode and cathode are, provide examples, and explain their roles in electrolysis. Additionally, we will explore the differences between anode and cathode, making the concepts clear and easy to grasp for students and enthusiasts alike.
What are Anode and Cathode?
Anode and cathode are terms used to describe the electrodes in an electrochemical cell. An electrode is a medium that allows electric current to flow into or out of a material, substance, or specific area. In simple terms:
- Anode: This is the point where electrons are lost (oxidation).
- Cathode: This is the point where electrons are gained (reduction).
These definitions hold true in both galvanic (voltaic) cells and electrolytic cells, but the direction of electron flow and the type of chemical reactions at each electrode can differ depending on the type of cell.
What is Anode?
The anode is the electrode where oxidation occurs. Oxidation is the process of losing electrons, which means the anode releases electrons into the external circuit. In a galvanic cell (like a battery), the anode is the negative terminal because it is the source of electrons. However, in an electrolytic cell, the anode is the positive terminal because it attracts anions (negative ions) from the electrolyte.
Examples of Anode
- Galvanic Cell: In a zinc-copper galvanic cell, the zinc electrode serves as the anode. Zinc loses electrons and undergoes oxidation, forming zinc ions (Zn²⁺).
- Electrolysis of Water: In the electrolysis of water, the anode is made of an inert material like platinum or graphite. At the anode, water molecules undergo oxidation, leading to the production of oxygen gas.
- Electroplating: In copper electroplating, the copper plate being used as the source of copper atoms is the anode. Copper atoms lose electrons and go into the solution as Cu²⁺ ions.
What is Cathode?
The cathode is the electrode where reduction occurs. Reduction is the process of gaining electrons, meaning the cathode receives electrons from the external circuit. In a galvanic cell, the cathode is the positive terminal because it receives electrons. In an electrolytic cell, the cathode is the negative terminal because it attracts cations (positive ions) from the electrolyte.
Examples of Cathode
- Galvanic Cell: In a zinc-copper galvanic cell, the copper electrode serves as the cathode. Copper ions (Cu²⁺) gain electrons and undergo reduction, depositing copper metal on the electrode.
- Electrolysis of Water: In the electrolysis of water, the cathode is typically made of platinum or graphite. Hydrogen gas is produced at the cathode through the reduction of water molecules.
- Electroplating: In copper electroplating, the object to be plated (like a spoon) serves as the cathode. Copper ions in the electrolyte receive electrons and form a thin layer of copper metal on the object’s surface.
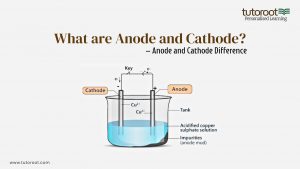
Anode and Cathode in Electrolysis
Electrolysis is a method that utilizes electrical energy to initiate a chemical reaction that wouldn’t occur on its own. This process is widely used in various industrial applications, such as the extraction of metals, electroplating, and the production of chlorine gas and caustic soda.
Basic Principle of Electrolysis
In an electrolytic cell, an external voltage source (like a battery) is connected to two electrodes submerged in an electrolyte solution. The electrolyte contains ions that can move to the electrodes, where they either gain or lose electrons, resulting in chemical reactions.
- Anode (Positive Electrode):
- Oxidation occurs at the anode.
- Negative ions (anions) in the electrolyte are attracted to the anode.
- At the anode, anions lose electrons (oxidation) and form neutral atoms or molecules.
- Cathode (Negative Electrode):
- Reduction occurs at the cathode.
- Positive ions (cations) in the electrolyte are attracted to the cathode.
- At the cathode, cations gain electrons (reduction) and form neutral atoms or molecules.
Importance of Electrolysis
Electrolysis plays a crucial role in modern industry and technology. It allows for the extraction of pure metals, the production of essential chemicals, and the enhancement of material properties through electroplating. Understanding the principles of electrolysis, particularly the roles of anode and cathode, is fundamental for students and professionals in chemistry, materials science, and engineering.
Step-by-Step Process of Electrolysis
- Application of Voltage: When the external voltage source is connected, an electric field is established between the anode and cathode.
- Movement of Ions: The electric field in the electrolyte causes ions to move towards the electrodes. Positive ions (cations) are attracted to the cathode, while negative ions (anions) are drawn to the anode.
- Oxidation at the Anode: At the anode, anions lose electrons (oxidation) and form neutral atoms or molecules. These may either dissolve in the electrolyte or be released as gases.
- Reduction at the Cathode: At the cathode, cations gain electrons (reduction) and form neutral atoms or molecules, which may deposit on the electrode or be released as gases.
Example: Electrolysis of Water
The electrolysis of water is a classic example that demonstrates the principles of electrolysis. In this process, water (H₂O) is decomposed into oxygen (O₂) and hydrogen (H₂) gases using an electric current. An electrolyte like sulfuric acid (H₂SO₄) or sodium hydroxide (NaOH) is added to increase the conductivity of water.
Setup:
- Anode: Made of an inert material like platinum or graphite, connected to the positive terminal of the power source.
- Cathode: Made of an inert material like platinum or graphite, connected to the negative terminal of the power source.
- Electrolyte: Dilute sulfuric acid or sodium hydroxide solution.
Reactions:
1. At the Anode (Oxidation):
\( 2 H_{2}O(I) \longrightarrow O_{2}(g)+4 H^{+} (aq)+4 e^{-}\)
Water molecules lose electrons (oxidation) to form oxygen gas and hydrogen ions.
2. At the Cathode (Reduction):
\(4 H^{+}(aq)+4 e^{-} \longrightarrow 2 H_{2}(g) \)
Hydrogen ions gain electrons (reduction) to form hydrogen gas.
Overall Reaction:
\( 2 H_{2}O(I) \longrightarrow 2 H_{2}(g)+ O_{2}(g) \)
This reaction shows that water decomposes into hydrogen gas at the cathode and oxygen gas at the anode.
Industrial Applications of Electrolysis
- Metal Extraction: Electrolysis is used to extract metals from their ores. For example, aluminum is extracted from bauxite ore using electrolysis in the Hall-Héroult process.
- Electroplating: Electrolysis is employed to coat objects with a thin layer of metal. This is used in jewelry making, manufacturing of electronic components, and corrosion protection.
- Production of Chemicals: Electrolysis is used to produce chemicals like chlorine, hydrogen, and sodium hydroxide. For example, the chlor-alkali process produces chlorine gas and caustic soda by electrolyzing brine (sodium chloride solution).
Difference Between Anode and Cathode
The key difference between anode and cathode are tabulated below,
| Parameter | Anode | Cathode |
| Type of Reaction | Oxidation (loss of electrons) | Reduction (gain of electrons) |
| Charge in Electrolytic Cell | Positive | Negative |
| Charge in Galvanic Cell | Negative | Positive |
| Attraction | Attracts anions (negative ions) | Attracts cations (positive ions) |
| Electron Flow | Electrons flow away from the anode | Electrons flow towards the cathode |
| Reaction Example | Zn → Zn²⁺ + 2e⁻ (oxidation) | Cu²⁺ + 2e⁻ → Cu (reduction) |
| Common Use | Source of electrons in batteries | Receiving electrons in batteries |
| Electroplating Role | Metal dissolves into the solution | Metal deposits onto the object |
| Gas Production (Electrolysis) | Oxygen gas is produced | Hydrogen gas is produced |
| Industrial Application | Extraction of metals from ores | Coating objects with metal layers |
Final Notes
Understanding the roles of anode and cathode is crucial in the study of electrochemistry and various industrial processes. The anode is where oxidation occurs, releasing electrons, while the cathode is where reduction occurs, gaining electrons. These concepts are integral to the functioning of batteries, electrolysis, and electroplating, making them fundamental knowledge for students and professionals in chemistry and related fields.
For a seamless understanding of various concepts, just like those explained above, explore the Tutoroot Blog section for simplified learning experiences. Elevate your comprehension of subjects and have your queries addressed through Tutoroot’s Chemistry Online Tuition. Start your learning journey with Tutoroot’s online home tuitions by scheduling a FREE DEMO session today.
FAQs
What is Cathode and Anode Charge?
In an electrolytic cell, the anode has a positive charge, and the cathode has a negative charge. Conversely, in a galvanic cell, the anode carries a negative charge, and the cathode carries a positive charge.
Give Cathode and Anode Symbol.
The symbol for the anode is often represented as A or + in an electrolytic cell and – in a galvanic cell. The symbol for the cathode is C or – in an electrolytic cell and + in a galvanic cell.
What is Cathode and Anode Difference?
The key difference lies in their functions: the anode is the site of oxidation, where electrons are lost, whereas the cathode is the site of reduction, where electrons are gained.
