What is Bohr’s Model of an Atom? – Diagram, Applications, Limitations
What is Bohr’s Atomic Model?
Neil Bohr proposed the Bohr;s model in 1915. Bohr’s atomic model was developed by a modification to Rutherford’s atomic model. Rutherford’s concept established the nuclear model of an atom, in which a positively charged nucleus is surrounded by negatively charged electrons. Thomson’s and Rutherford’s atomic models failed to answer any queries about an atom’s energy and stability. In this aspect, Bohr’s model or Bohr’s atomic model shows an advancement in the study and findings

As per Bohr’s atomic model, the atomic structure model was amended. This amendment indicated that electrons travel in fixed orbitals (shells) and not everywhere in between, and that each orbit (shell) has a fixed energy. Rutherford described an atom’s nucleus, and Bohr (as per the Bohr’s model), expanded on that concept to include electrons and their energy levels.
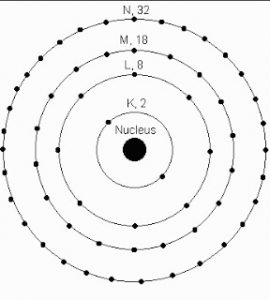
Diagram of Bohr’s Model
The Bohr’s model diagram is shown below,
Introduction Bohr’s Atomic Model and a detailed explanation
Niels Bohr in 1913 went on to propose a model that showcased an atom as a minute, small, and positively charged nucleus that is electronics surrounding it. These electrons, as per Bohr’s atomic model move in orbits that are circular. The electrons travel around the nucleus and are positively charged. As per Bohr’s model, these can be planets around the sun in the solar system. This is according to the attraction by electrostatic forces, a profound and timeless observation advocated under Bohr’s model or Bohr’s atomic model.
Bohr’s model was a primary improvement of Rutherford’s atomic model addressing certain gaps
Some of the important features of Bohr’s atomic model are:
Electrons revolve around the nucleus and that too in a stable orbit. This happens without them emitting radiant energy.
Be it an orbit or an energy level, as per Bohr’s model, they are represented as K, L, M, N shells. The ground state of an electron refers to its lowest level of energy. This comes from the fact that every orbit possesses a specific energy termed as level or shell, a vital point in Bohr’s atomic model.
The energy emission in an electron happens when it moves from one orbit to another.
The energy absorbed or emitted is the same as the difference seen in energies between the two levels. This is explained in Plank’s equation (E1, E2).
\(\Delta E= E_{2} – E_{1}\)
Where:
ΔE = energy absorbed or emitted
h= Plank’s constant
𝜈= frequency of electromagnetic radiation emitted or absorbed
The angular momentum of an electron that moves in energy shells is shown as:
\(m_{e}vr= \frac{nh}{2 \pi }\)
where:
n= number of corresponding energy shell; 1, 2, 3…
me= mass of the electron
v= velocity
r=radius
h= Plank’s constant
Bohr’s Model
Distribution of electrons in orbits or shells
The distribution of electrons in orbits and shells, as per Bohr’s atomic model, is measured in a formula: 2n2 where ‘n’ stands for the number of orbits.
In a K shell, that is the first orbit, the number of electrons is calculated as 2n2= 2 x 12 = 2. Therefore, we arrive at the maximum number of electrons in the orbit one as 2
In the same fashion, the number of electrons in the second orbit, known as the L shell is 2 x 22 = 8. The maximum number of electrons here is 8
Postulates of Bohr’s Model
The Bohr’s model postulates are given below,
- In an atom, electrons (negatively charged particles) rotate around the positively charged nucleus in a predetermined circular route known as orbits or shells.
Each orbit or shell has a predetermined energy, and these circular orbits are referred to as orbital shells. - The quantum number n=1, 2, 3… represents the energy levels. This quantum number range series is on the nucleus side, with n=1 starting with the lowest energy level. The orbits n=1, 2, 3, 4… are designated as K, L, M, N…. shells, and an electron is said to be in the ground state when it reaches the lowest energy level.
- Electrons in an atom gain energy to go from a lower energy level to a higher energy level, and electrons lose energy to move from a higher energy level to a lower energy level.
Limitations of Bohr’s Atomic Model
Bohr Atomic Model comes with limitations such as:
- It goes against the Heisenberg Uncertainty Principle: Under Bohr’s model, electrons are considered to possess a defined radius and orbit, which are position and momentum at the same time. This is not at all possible as per Heisenberg.
- The Bohr’s atomic model boasts of accurate observations for atoms that are smaller in size. These may be like hydrogen, but in the case of larger atoms, the spectral predictions have been ordinary
- Bohr’s atomic model cannot talk about the Zeeman effect where the spectral line is divided into multiple components amid a magnetic field.
- Bohr’s model also fails to touch upon the Stark effect where a spectral line is split up into fine lines in an electric field.
Applications of Bohr’s Model of an Atom
- Bohr’s theory applies to hydrogen-like substances with only one electron, such as Li2+. Each Li2+ and H-atom has only one electron. He and He2+ have two and zero electrons, respectively.
Final Notes
Now that you know What Is Bohr’s Atomic Model? You may learn more about the intriguing aspects of atoms and other related subjects by exploring our website. Join today to learn from experts at Tutoroot, where you can experience the best online interactive classes.
FAQ
What is Bohr’s model of the atom?
Niels Bohr in 1913 went on to propose a model that showcased an atom as a minute, small, and positively charged nucleus that is electronics surrounding it. These electrons, as per Bohr’s atomic model move in orbits that are circular. The electrons travel around the nucleus and are positively charged. As per Bohr’s model, these can be planets around the sun in the solar system. This is according to the attraction by electrostatic forces, a profound and timeless observation advocated under Bohr’s model or Bohr’s atomic model.
Bohr’s model was a primary improvement of Rutherford’s atomic model addressing certain gaps
Why did the Bohr Model fail?
Bohr’s atomic model boasts of accurate observations for atoms that are smaller in size. These may be like hydrogen, but in the case of larger atoms, the spectral predictions have been ordinary.
What are Bohr’s 3 postulates?
- In an atom, electrons (negatively charged particles) rotate around the positively charged nucleus in a predetermined circular route known as orbits or shells.
- Each orbit or shell has a predetermined energy, and these circular orbits are referred to as orbital shells.
- The quantum number n=1, 2, 3… represents the energy levels. This quantum number range series is on the nucleus side, with n=1 starting with the lowest energy level. The orbits n=1, 2, 3, 4… are designated as K, L, M, N…. shells, and an electron is said to be in the ground state when it reaches the lowest energy level.
- Electrons in an atom gain energy to go from a lower energy level to a higher energy level, and electrons lose energy to move from a higher energy level to a lower energy level.
How many numbers of Electrons are present in K, L, M, and N Shells?
The maximum number of electrons in the shells K, L, M, and N are 2, 8, 18, and 32, respectively.
