What is Aldol Condensation? – Reaction, Mechanism, Example
The term aldol condensation is used for the union of two or more molecules of the same or different compounds to form a new substance with or without elimination of a smaller molecule like H₂O, HCL, NH3, C₂H₅OH, etc.
Aldehydes and ketones containing at least one α–hydrogen atom when treated with dilute alkali, e.g. NaOH, Ba (OH)₂, K₂CO₃, etc., undergo self-condensation to form β-hydroxy aldehydes and β-hydroxy ketones. Since β-hydroxy aldehydes possess both aldehydic and alcoholic groups, they are commonly known as aldols. Therefore, the reaction is known as aldol condensation. For example, acetaldehyde undergoes aldol condensation as follows:
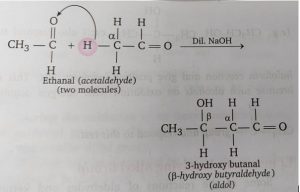
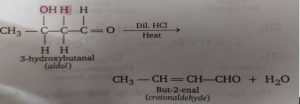
The process of aldol condensation i.e., β-hydroxy aldehydes or β-hydroxy ketones undergoes dehydration very easily when heated in the presence of dilute acid to form unsaturated carbonyl compounds. This is because the unsaturated carbonyl compound thus formed is quite stable due to conjugation. For example, the aldol (3-hydroxybutanal) formed in the above reaction on heating gives crotonaldehyde which is a stable compound.
It is to be noted that aldol condensation is undergone by only those aldehydes or ketones that possess at least one α–hydrogen atom. Those containing no α–hydrogen atom do not undergo aldol condensation. Thus, benzaldehyde (C6H5CHO), benzophenone ((C₆H₅)₂CO,), trimethyl acetaldehyde [ (CH3)3CCHO], etc., do not give this reaction. However, formaldehyde which possesses no α-hydrogen atom may undergo aldol condensation under suitable conditions.
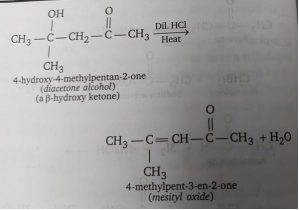
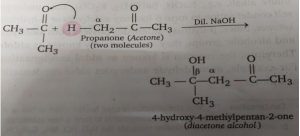
Acetone undergoes aldol condensation and the dehydration of the condensation product i.e., β-hydroxy ketone) as follows.
Aldol Condensation Reaction
Aldol condensation is a chemical process in which an enolate ion combines with a carbonyl molecule to make a β-hydroxy ketone or β-hydroxy aldehyde, which is then dehydrated to produce a conjugated enone. Aldol condensation is necessary in organic synthesis because it creates a channel for the creation of carbon-carbon bonds.

General Aldol Condensation Reaction
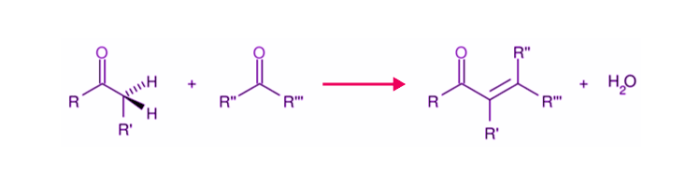
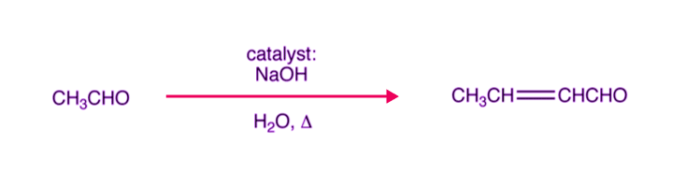
A common example of base-catalyzed aldol condensation is shown below, where the catalyst is typically a hydroxide ion.
Mechanism of Aldol Condensation
Aldol condensation takes place through the following three steps:
OH negative ion from the dilute alkali attacks the first molecule of aldehyde and extracts the α–hydrogen atom present in it to form an enolate ion which is resonance stabilized.
The enolate ion thus formed acts as a nucleophile and attacks the positively charged carbon atom of the carbonyl group of the second molecule of the aldehyde to form an anion.
The anion this formed abstract a proton (H+) from water to form aldol.
Step-1
In the reversed order, the hydroxide ion deprotonates the aldehyde.
Step-2
Enolate ion 1 reacts with the unreacted aldehyde.
Step-3
Water protonates alkoxide ion 2.
Step-4
Hydroxide ion converts a little quantity of aldol into an enolate ion (4).
Step-5
Enolate Ion (4) loses a hydroxide ion.
What is Cross Aldol Condensation?
The crossing aldol condensation reaction occurs with two different motes of an aldehyde or ketone condense in a protic liquid similar to water or alcohol. Crossed aldol condensation occurs when condensation occurs between two distinct carbonyl molecules. Both aldehydes with alpha hydrogens can create carbanions and behave as carbanion acceptors. As a result, a combination of four products of limited synthetic value is generated.
If one of the aldehydes does not contain alpha hydrogen, it can only act as a carbanion acceptor. In this situation, just two goods are produced. An aromatic aldehyde with no alpha position is a typical substrate for the crossing aldol reaction. Furthermore, the first condensation product dehydrates
quickly, resulting in the creation of the, α, β – unsaturated ketone and preventing the retro-aldol reaction.
Crossed Aldol Condensations Using Weak Bases
Crossed aldol reactions are possible with weak bases such as hydroxide or an alkoxide when one carbonyl reactant does not have a hydrogen. A reactant without a hydrogen cannot self-condense because it cannot form an enolate. We avoid self-condensation of the other reactant, that which has a hydrogen, by adding it slowly to a solution of the first reactant and the base. Under these conditions, the concentration of the reactant with a hydrogen is always low, and it is present mostly in its enolate form. The main reaction that takes place is between this enolate and the carbonyl compound that has no a hydrogen.
Example of aldol condensation
Some of the useful crossed aldol condensation reactions involving benzaldehyde are:
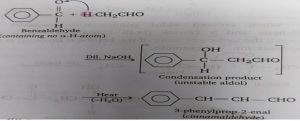
Reaction with acetaldehyde: Benzaldehyde undergoes crossed condensation with acetaldehyde to form an unstable aldol which on heating decomposes to give cinnamaldehyde.
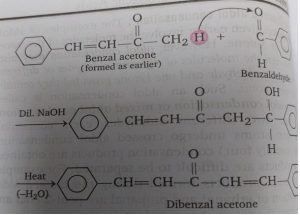
 Reaction with acetone: Similarly, when acetone is created with benzaldehyde in the presence of dilute NaOh, a condensation product is obtained which on heating gives benzal acetone.
Reaction with acetone: Similarly, when acetone is created with benzaldehyde in the presence of dilute NaOh, a condensation product is obtained which on heating gives benzal acetone.
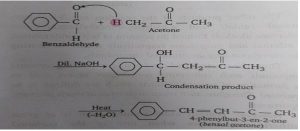
If benzaldehyde is used in excess, dibenzyl acetone is obtained because benzal acetone formed in the reaction further condenses with benzaldehyde.
Types of Condensation
- Enolate produced by anhydride in a Perkin reaction is aromatic.
- Two ester compounds are present in a Claisen condensation.
- An aliphatic nitro molecule and an aldehyde are used in the Henry reaction.
- Dieckmann condensation involves the presence of two ester groups in the same molecule, resulting in a cyclic molecule.
- Water is removed by nucleophilic displacement in Japp-Maitland condensation.
Cannizaro reaction
Aldehydes which do not have an -hydrogen atom, undergo self-oxidation and reduction (disproportionation) on treatment with concentrated alkali.
HCHO+HCHO conc.NaOH−−−−−−−→ HCOOH+HCOONa
(c) Cross aldol condensation
CH3CHO+CH3CH2CHO 1 NaOH−−−−−→2Δ CH3−CH=CH−CHO+
CH3−CH2−CH=C−CHO
|
CH3
CH3−CH=C−CHO + CH3CH2−CH=CH−CHO
|
CH3
When an aldehyde containing no α-hydrogen atom is heated with a concentrated solution of sodium or potassium hydroxide solution, it undergoes disproportionation, i.e., self-oxidation reduction. Two molecules of the aldehyde take part in the reaction; one gets oxidised to a carboxylic acid at the expense of the other which gets reduced to an alcohol. The carboxylic acid formed in the reaction combines with the alkali to form the corresponding salt.
The reaction involving simultaneous oxidation and reduction of an aldehyde containing no α-hydrogen atom is called Cannizzaro reaction.
Electrophilic Substitution Reaction of Benzene Ring
Aromatic aldehydes and ketones undergo usual electrophilic substitution reactions of benzene ring, e.g., halogenation, nitration and sulphonation. Since, the carbonyl group present in the aromatic aldehydes and ketones is m-directing, the substitution takes place predominantly at the m-position of the ring. Therefore, the electrophilic substitution reactions in aromatic aldehydes and ketones are slow. Moreover, due to certain side reactions, e.g., oxidation, the yield of the substitution products is poor.
Halogenation of ketone
Aromatic aldehydes and ketones undergo nuclear halogenation (substitution in the ring) with considerable difficulty because side-chain halogenation occurs faster. For example, benzaldehyde reacts with chlorine even in the absence of a halogen carrier to form benzoyl chloride.
Similarly, when acetophenone is treated with bromine in ether at 273 K in the presence of anhydrous aluminium chloride, bromination occurs in the side chain and phenacyl bromine is obtained.
The vapours of phenacyl bromine attack the nose, throat and lungs. Therefore, it is used as a tear gas to disperse the mob.
When acetophenone is treated with bromine in the presence of excess aluminium chloride, m-bromoacetophenone is obtained. The yield of the reaction is 75%. The substitution takes place under swamping catalytic conditions when an excess of aluminium chloride forms a complex with the carbonyl group.
Since nuclear halogenation in aromatic aldehydes and ketones is quite difficult, their nuclear halogen derivatives are prepared by indirect methods.
Final Notes
Use the book Organic Chemistry by Morrison and Boyd to have a deeper knowledge of this concept. Solving previous year’s JEE papers would also help you or get expert help from Tutoroot.
FAQ’s
Define aldol condensation with an example.
The term condensation is used for the union of two or more molecules of the same or different compounds to form a new substance with or without elimination of a smaller molecule like H₂O, HCL, NH3, C₂H₅OH, etc.
Aldehydes and ketones containing at least one α–hydrogen atom when treated with dilute alkali, e.g. NaOH, Ba (OH)₂, K₂CO₃, etc., undergo self-condensation to form β-hydroxy aldehydes and β-hydroxy ketones. Since β-hydroxy aldehydes possess both aldehydic and alcoholic groups, they are commonly known as aldols. Therefore, the reaction is known as aldol condensation. For example, acetaldehyde undergoes aldol condensation as follows:
Explain aldol condensation
Aldol condensation is a chemical process in which an enolate ion combines with a carbonyl molecule to make a β-hydroxy ketone or β-hydroxy aldehyde, which is then dehydrated to produce a conjugated enone. Aldol condensation is necessary in organic synthesis because it creates a channel for the creation of carbon-carbon bonds.
