What is Isomerism? Definition, Types, Examples

While studying Chemistry, students will come across Isomers, or about the compounds Isomerism. Besides, many students often have a hard time trying to understand this topic, thus helping them out. We have put together a detailed guide about Isomerism, its types, as well as examples. To learn more about the concept, go through the article provided below.
What Is Isomerism?
Firstly, let us understand what Isomerism is. Chemical compounds are known to have similar chemical formulae, but they differ greatly in the arrangement of atoms for the compound as well as the arrangement of atoms. This phenomenon is called isomerism, and the compounds which express this property are called Isomers.
Types of Isomerism
Now that you have a basic idea of what Isomerism is? And What Isomers are? Let us now talk about the different types of isomerism that are commonly found such as,
Structural Isomerism
Structural Isomerism is also called Constitutional Isomerism, mainly because atoms observed in the molecule, and functional groups, are usually linked differently from one another. Although different types of structural isomers are designated IUPAC names, whether they are from the same functional group or not, will depend on the type of isomers.
Besides, this, there are multiple types of structural isomerism, which we will explain briefly here in the below section.
Chain Isomerism
The isomers whose components have different branched structures, which is why they are also called Skeletal Isomers. Given below are some common examples of Chain Isomerism.
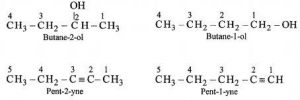
Position Isomerism
As the name itself suggests, the compounds where this type of isomerism is found, usually have different substituent atoms that are placed in different positions.
Functional Isomerism
These compounds generally have the same chemical formula, but the functional groups in each compound differ from one another.
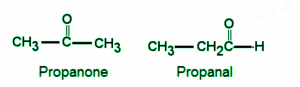
Ring Chain Isomerism
This is a unique isomerism where two isomers have different structures, one open-chained and one ring structure. Which in turn causes the number of pi bonds in these compounds to be different.

Metamerism
If the compound consists of different alkyl chains on either side of the functional group, then this phenomenon is called Metamerism.

Tautomerism
Another unique isomerism is where the compounds only have differences in the positions of either protons or electrons. Another unique feature of these isomers is that they easily exist together in equilibrium, which is they can easily interchange.
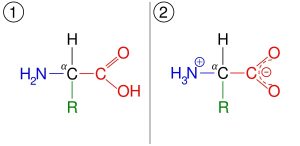
Stereo Isomerism
The compounds have the same chemical formula but they have different orientations, as they are generally observed in molecules existing in three-dimensional space. And as you can guess, the compounds where the phenomenon is observed, are referred to as Stereo Isomers, which are further differentiated into two types,
-
Geometric Isomerism
The isomers that observe the spatial arrangement of atoms in 3d space are generally called geometric isomers, and this phenomenon is referred to as cis-trans or geometric isomerism.
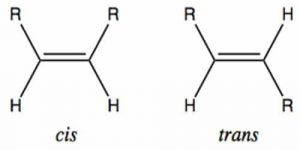
-
Optical Isomerism
The compounds have similar bonds; however, the spatial arrangement of atoms is not the same, which in turn results in non-superimposable mirror images.
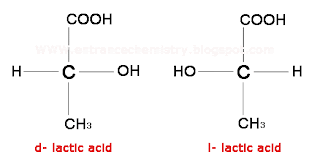
Ionization Isomerism
Similar to the other isomers explained above, these isomers also show similar composition, however, the compound in this isomer, produces different ions, while being in a solution. For instance, when you observe a counter ion, in a complex salt it can be a potential ligand, thus displacing the counter ion, and converting it to the ligand.
Conclusion
Here in the above article, we have provided a comprehensive description of Isomerism, Types of Isomerism, Examples of Isomerism, and many more. If you want to learn more about any difficult topics in Chemistry, then it would be a good idea for the students to Join the Online Interactive Classes offered by our Tutoroot platform as they can access various benefits, which will help them get better ranks and scores in the examinations.
For more simplified explanations like the one above, visit the chemistry blogs on the Tutoroot website. Elevate your learning with Tutoroot’s personalised Chemistry online tuition. Begin your journey with a FREE DEMO session and discover the advantages of one-on-one online guidance.

