What is Spectrophotometer Principle? – Applications, Diagram
Introduction to Spectrophotometer
In the realm of scientific instruments, one powerful tool that stands out for its ability to analyze and quantify the properties of substances is the spectrophotometer. This ingenious device makes use of the principles of optics to measure the amount of light absorbed or transmitted by a sample, enabling scientists to delve into the fascinating world of molecular interactions. In this article, we will explore the fundamental principles behind the spectrophotometer and its various applications, shedding light on its instrumental setup, essential equations, and the significance of Beer’s and Lambert’s laws.
Introduction to Optics
Before diving into the intricacies of spectrophotometry, it is essential to grasp the basics of optics. Optics is the branch of physics concerned with the behavior and properties of light, including its interaction with various materials. Understanding concepts such as absorption, transmission, reflection, and refraction allows us to comprehend the working principles of spectrophotometers effectively.
What is a Spectrometer?
A spectrometer is a critical component in the spectrophotometer’s setup. It is an instrument that helps disperse light into its component wavelengths, allowing for precise analysis of the light spectrum. By employing prisms or gratings, spectrometers split light into its constituent colors, revealing its unique fingerprint of wavelengths.
What is a Spectrophotometer?
A spectrophotometer, on the other hand, utilizes the outputs from a spectrometer and combines them with advanced electronic systems to measure and quantify the intensity of light at different wavelengths. This measurement enables scientists to determine the extent of absorption or transmission of light by a sample substance. Spectrophotometers have become indispensable tools across various scientific disciplines, from chemistry and biology to environmental science and medicine.
What is the Spectrophotometer Principle?
The spectrophotometer principle revolves around the fundamental concept that substances selectively absorb or transmit light at specific wavelengths. This absorption or transmission behavior is governed by the chemical structure and composition of the substance. Spectrophotometers exploit this principle by measuring the intensity of light before and after interacting with a sample, allowing scientists to determine its absorption or transmission characteristics.
Sounds difficult? Let’s understand in another way. To understand the principle behind a spectrophotometer, we need to delve into some fundamental concepts. At its core, a spectrophotometer operates on the principle of light absorption and transmission. It measures the amount of light that passes through a sample at different wavelengths and compares it to the amount of light that passes through a reference solution or blank.
Spectrophotometer Instrumentation
To grasp the intricacies of a spectrophotometer’s operation, let’s dive into its core instrumentation. The main components of a spectrophotometer include a light source, a monochromator or a spectrometer, a sample holder, and a detector. These components work in unison to produce accurate and reliable measurements of light absorption or transmission.
Spectrophotometer Instrumentation Diagram
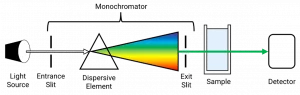
To visualize the setup of a spectrophotometer, consider the following diagram:
- Light Source: This component emits a broad spectrum of light, which serves as the input for the subsequent analysis.
- Monochromator or Spectrometer: The incoming light passes through this unit, which disperses the light into individual wavelengths and isolates the desired range for analysis.
- Sample Holder: This component holds the sample under investigation, allowing the light to interact with it and subsequently determine its properties.
- Detector: The detector, often a photodiode or a photomultiplier tube, quantifies the intensity of light transmitted or absorbed by the sample across the selected range of wavelengths.
Spectrophotometer Instrumentation Process
The process of measuring light absorption or transmission with a spectrophotometer involves the interaction of light with a sample, detection of the transmitted or absorbed light, and subsequent data analysis. To better understand this process, let’s delve into each step:
- The light source emits a beam of light that passes through a monochromator, which selects a specific wavelength.
- This monochromatic light then travels through the sample solution in the cuvette.
- The detector measures the intensity of the light that exits the sample.
- The spectrophotometer compares this intensity to the intensity of light passing through a blank or reference solution. The blank serves as a baseline to account for any absorption or scattering by the solvent or container.
- By calculating the difference between the two intensities, the spectrophotometer determines how much light was absorbed by the sample.
- This absorption data is then used to quantify the concentration of a substance in the sample, as different substances absorb light at different wavelengths and in varying amounts.
Additionally, the spectrophotometer instrumentation process involves the use of advanced electronics, such as amplifiers and filters, to ensure accurate and reliable measurements.
Beer’s Law
One of the fundamental principles guiding the quantification of light absorption is Beer’s law, formulated by August Beer. This law states that the amount of light absorbed by a sample is directly proportional to its concentration. Mathematically, Beer’s law is expressed as:
A = ε * c * l
Where:
- A represents the absorbance of the sample
- ε symbolizes the molar absorptivity, a substance-specific constant
- c denotes the concentration of the sample
- l represents the path length, indicating the distance traveled by the light through the sample
Beer’s law finds extensive application in various quantitative analyses, making it a cornerstone of spectrophotometry.
Lambert’s Law
Lambert’s law, proposed by Johann Lambert, is another principle fundamental to spectrophotometry. This law states that the absorbance of a substance is directly proportional to its path length and the concentration. Mathematically, Lambert’s law is expressed as:
A = log₁₀ (Io / I)
Where:
- A represents the absorbance of the sample
- Io signifies the intensity of incident light
- I denotes the intensity of transmitted light
Lambert’s law provides valuable insights into the relationship between light absorption, concentration, and path length, allowing for accurate calculations in spectrophotometric analyses.
Applications of Spectrophotometer
Spectrophotometers find widespread applications across various scientific domains. Some key areas where spectrophotometers are utilized include:
- Environmental Analysis: Spectrophotometers enable the analysis of pollutants, such as heavy metals and organic compounds, in environmental samples like water, soil, and air.
- Biomedical Research: In the field of medicine and biology, spectrophotometry aids in DNA quantification, enzyme activity measurements, and various diagnostic tests.
- Pharmaceutical Analysis: Spectrophotometers play a crucial role in pharmaceutical research by facilitating drug stability studies, drug dissolution testing, and analysis of drug impurities.
- Quality Control: Spectrophotometric techniques are employed in numerous industries, including food and beverage, to ensure product quality and monitor the concentration of key components.
These examples merely scratch the surface of the wide array of applications that spectrophotometers offer. Their versatility and precision make them indispensable tools in scientific research and analysis.
Final Notes
The spectrophotometer stands as a remarkable instrument that harnesses the principles of optics, enabling scientists to explore the fascinating world of light absorption and transmission. By understanding the spectrophotometer’s underlying principles, instrumentation, equations like Beer’s and Lambert’s laws, and its diverse applications, we can appreciate the essential role it plays in unraveling the intricate properties of substances.
We believe that this article has successfully answered all your queries regarding meristematic tissue. If you’d like to delve into more simplified concepts, please visit our Tutoroot blog section. Moreover, if you’re looking for outstanding online tutoring to improve your academic achievements, Tutoroot is the perfect option for you. Don’t wait; click here now to schedule a FREE DEMO with our exceptional faculty members in the respective field.
FAQs
Define Spectrophotometer Principle
The spectrophotometer principle revolves around the concept that substances selectively absorb or transmit light at specific wavelengths. By measuring the intensity of light before and after it interacts with a sample, spectrophotometers allow scientists to determine the sample’s absorption or transmission characteristics, providing valuable insights into its composition and other properties.
What is the difference between a Spectrometer and a Spectrophotometer?
While both spectrometers and spectrophotometers deal with the analysis of light, they serve different purposes. A spectrometer disperses light into its component wavelengths, concentrating on the qualitative aspects of the spectrum. On the other hand, a spectrophotometer utilizes the outputs from a spectrometer to quantitatively measure the intensity of light at specific wavelengths, focusing on the interaction of light with substances and providing valuable data on absorption or transmission behaviors.
