What is Aufbau Principle? – Definition, Diagram
The Aufbau Principle is a fundamental concept in chemistry that helps us understand and predict the electronic configuration of atoms. In this blog post, we will delve into the details of the Aufbau Principle, exploring its definition, diagram, formula, and practical applications. By the end of this post, you will have a clear understanding of how the Aufbau Principle works and its significance in the world of chemistry.
Aufbau Principal Definition
The Aufbau Principle, also known as the building-up principle, states that electrons fill atomic orbitals to increase energy. In simpler terms, electrons occupy the lowest energy orbitals first before moving on to higher energy ones. This principle is based on the idea that electrons strive to achieve the most stable configuration possible.
Aufbau Principal Diagram
To visualize the Aufbau Principle, we can refer to a diagram called the Aufbau diagram. This diagram represents the order in which electrons fill atomic orbitals. Starting with the lowest energy level, which is the 1s orbital, electrons progressively fill higher energy levels, following a specific pattern.
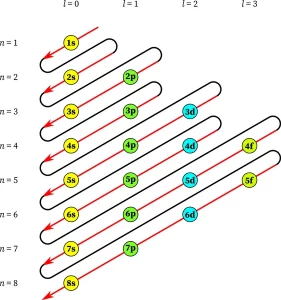
Electronic Configuration Using Aufbau Principle
The Aufbau Principle provides us with a systematic approach to determining the electronic configuration of atoms. By knowing the number of electrons an atom has, we can fill the orbitals step by step, adhering to the Aufbau Principle.
Let’s take the example of carbon, which has an atomic number of 6.
- The first two electrons occupy the 1s orbital.
- The next two electrons fill the 2s orbital.
- The remaining two electrons go into the 2p orbital, with one electron in each of the three available 2p orbitals.
By following this process, we arrive at the electronic configuration of carbon: 1s² 2s² 2p².
Silent Features of the Aufbau Principle
The Aufbau Principle comes with several silent features that are worth mentioning:
- Orderly filling: The principle ensures that electrons are filled in a methodical and organized manner, following a sequence of increasing energy levels.
- Predictability: With the Aufbau Principle, we can easily predict the electronic configuration of atoms without the need for complex calculations.
- Stability: By filling orbitals in a specific order, the Aufbau Principle helps atoms achieve their most stable configuration, minimizing energy.
Aufbau Principal Example
Let’s consider another example to solidify our understanding of the Aufbau Principle. We will look at the electronic configuration of nitrogen, which has an atomic number of 7.
- The first two electrons fill the 1s orbital.
- The next two electrons occupy the 2s orbital.
- One electron goes into each of the three available orbitals.
Following this process, we obtain the electronic configuration of nitrogen: 1s² 2s² 2p³.
Limitations of the Aufbau Principle
While the Aufbau Principle serves as a useful guideline, there are certain exceptions and limitations to keep in mind:
- Crisscrossing orbitals: In some cases, due to the interaction between different energy levels, the order in which orbitals are filled may deviate from the expected pattern.
- Half-filled and fully-filled orbitals: At times, electrons may prefer to occupy half-filled or fully-filled orbitals, resulting in deviations from the standard filling order.
It is important to be aware of these exceptions when applying the Aufbau Principle to specific elements.
Final Notes
In conclusion, the Aufbau Principle is a fundamental concept that helps us understand how electrons fill atomic orbitals. By following the principle, we can predict the electronic configuration of atoms and gain valuable insights into their stability. Despite its limitations, the Aufbau Principle remains a vital tool in the field of chemistry, enabling scientists to unravel the complexities of the atomic world.
Keen on effortlessly mastering concepts, much like the detailed explanation provided above? Dive into our Tutoroot Blog section for simplified learning. Enhance your comprehension of subjects and have your questions answered through Tutoroot’s online tuition. Try out Tutoroot’s online home tuitions now by scheduling a FREE DEMO session.
FAQs
Q: Explain the Aufbau Principle.
The Aufbau Principle states that electrons fill atomic orbitals in order of increasing energy. This means that electrons occupy the lowest energy orbitals first before moving on to higher energy ones.
Q: What is the definition of the Aufbau Principle?
The Aufbau Principle, also known as the building-up principle, refers to the principle that electrons fill atomic orbitals in a specific order, starting from the lowest energy level and progressing to higher energy levels.
Q: State the Aufbau Principle.
The Aufbau Principle states that electrons fill atomic orbitals in order of increasing energy.
Q: State and explain the Aufbau Principle.
The Aufbau Principle states that electrons occupy atomic orbitals in a systematic and orderly manner, beginning with the lowest energy level and gradually filling higher energy levels. This principle ensures that atoms achieve their most stable configurations.
Q: What is the exception to the Aufbau Principle?
There are exceptions to the Aufbau Principle, particularly when it comes to the ordering of electron filling due to orbital interactions and preferences for half-filled or fully-filled orbitals.
