What is Decomposition Reaction? – Formula, Examples, Reaction
Decomposition reactions are fundamental chemical processes essential for understanding various natural and industrial phenomena. These reactions involve breaking down a single compound into two or more simpler substances. Decomposition reactions are vital in numerous fields, including chemistry, biology, and environmental science. This article will explore decomposition reactions, their formulas, examples, and different types, making it easy for students to grasp the concept.
Let’s Understand What is Decomposition Reaction?
A decomposition reaction is a chemical process in which a single compound is split into two or more simpler substances. These reactions are generally endothermic, meaning they require energy input to occur. The energy needed can come from various sources, such as heat, light, or electricity. Decomposition reactions are crucial in various chemical processes, including the breakdown of organic matter, industrial manufacturing, and even biological systems.
Decomposition Reaction Formula
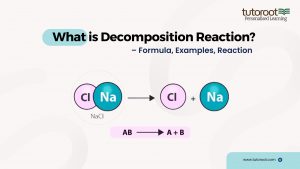
The general formula for a decomposition reaction can be represented as:
\(AB \longrightarrow A+B\)
In this formula, AB is the compound that breaks down into its constituent elements or simpler compounds A and B. This equation can vary depending on the specific substances involved in the reaction.
Decomposition Reaction Examples
Let’s explore a few examples to better understand decomposition reactions:
- Decomposition of Hydrogen Peroxide:
In this reaction, hydrogen peroxide decomposes into water and oxygen gas. This reaction is often catalyzed by the presence of a substance like manganese dioxide.
- Decomposition of Calcium Carbonate:
When calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide. This reaction is crucial in limestone production in the cement industry.
Types of Decomposition Reaction
Decomposition reactions can be classified into several types based on the source of energy required for the reaction. Understanding these classifications helps in comprehending the various ways decomposition reactions can occur.
Classification of Decomposition Reaction
Decomposition reactions can be broadly classified into the following types:
- Thermal Decomposition
- Electrolytic Decomposition
- Photo Decomposition
- Double Decomposition
Thermal Decomposition
Now let’s look at the Thermal Decomposition reaction,
What is a Thermal Decomposition Reaction?
Thermal decomposition is a type of decomposition reaction where heat is used to break down a compound into simpler substances. These reactions typically occur at high temperatures and are common in various industrial processes.
Thermal Decomposition Reaction Example
One common example of thermal decomposition is the decomposition of calcium carbonate:
\(CaCO_{3} \longrightarrow CaO+C O_{2}\)Here, calcium carbonate decomposes into calcium oxide (lime) and carbon dioxide gas when heated.
Electrolytic Decomposition
Here is the complete description of electrolytic decomposition,
What is Electrolytic Decomposition?
Electrolytic decomposition, also known as electrolysis, involves using electrical energy to break down a compound into its components. This type of reaction is widely used to extract and purify metals from their ores.
Electrolytic Decomposition Example
An example of electrolytic decomposition is the electrolysis of water:
\(2 H_{2} O \longrightarrow 2 H_{2} + O_{2}\)In this process, water is decomposed into hydrogen and oxygen gases by passing an electric current through it.
Photo Decomposition
What is Photo Decomposition?
Photo decomposition, or photolysis, is a type of decomposition reaction where light energy, usually in the form of ultraviolet light, is used to break down a compound. This reaction is significant in environmental and biological processes.
Photo Decomposition Example
A well-known example of photodecomposition is the decomposition of silver chloride:
\(2AgCl \longrightarrow 2Ag+ Cl_{2} \)In this reaction, silver chloride decomposes into silver and chlorine gas when exposed to light.
What is a Double Decomposition Reaction?
Double decomposition reactions, also known as double displacement reactions, involve the exchange of ions between two compounds to form new compounds. These reactions often occur in aqueous solutions and can result in the formation of a precipitate, a gas, or a weak electrolyte.
Final Notes
Decomposition reactions are integral to various natural and industrial processes. By understanding the different types of decomposition reactions and their specific examples, students can gain a deeper insight into the fundamental concepts of chemistry. Whether it’s the breakdown of hydrogen peroxide or the thermal decomposition of calcium carbonate, these reactions highlight the diverse ways in which compounds can be transformed into simpler substances.
Looking for clear explanations of various concepts, just like the one you read? Visit the Tutoroot Blog section for simplified learning experiences. Enhance your understanding of subjects and get your questions answered with Tutoroot’s chemistry online tuition. Start your journey with Tutoroot’s online home tuitions by scheduling a FREE DEMO session today.
FAQs
What is Physical Decomposition?
Physical decomposition refers to the physical breakdown of a substance into smaller pieces without changing its chemical composition. This process involves physical forces like grinding, cutting, or pulverizing.
Define Chemical Decomposition
Chemical decomposition is a process where a chemical compound is broken down into simpler substances through chemical reactions. This process involves breaking chemical bonds and often requires an input of energy.
What is the Formula for the Decomposition Reaction?
The general formula for a decomposition reaction is:
\(AB \longrightarrow A+B\)
Here, AB represents the compound that decomposes into simpler substances A and B.
